Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury
- PMID: 9133375
- PMCID: PMC6573673
- DOI: 10.1523/JNEUROSCI.17-10-03503.1997
Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury
Abstract
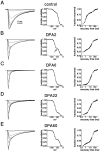
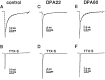
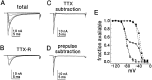
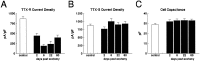
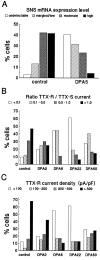
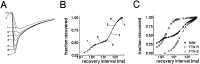
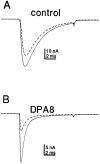
Clinical and experimental studies have shown that spinal sensory neurons become hyperexcitable after axonal injury, and electrophysiological changes have suggested that this may be attributable to changes in sodium current expression. We have demonstrated previously that sodium channel alpha-III mRNA levels are elevated and sodium channel alpha-SNS mRNA levels are reduced in rat spinal sensory neurons after axotomy. In this study we show that small (C-type) rat spinal sensory neurons express sodium currents with dramatically different kinetics after axotomy produced by sciatic nerve ligation. Uninjured C-type neurons express both slowly inactivating tetrodotoxin-resistant (TTX-R) sodium current and a fast-inactivating tetrodotoxin-sensitive (TTX-S) current that reprimes (recovers from inactivation) slowly. After axotomy, the TTX-R current density was greatly reduced. No difference was observed in the density of TTX-S currents after axotomy, and their voltage dependence was not different from controls. However, TTX-S currents in axotomized neurons reprimed four times faster than control TTX-S currents. These data indicate that axotomy of spinal neurons is followed by downregulation of TTX-R current and by the emergence of a rapidly repriming TTX-S current and suggest that this may be attributable to the upregulation of a sodium channel isoform that was unexpressed previously in these cells. These axotomy-induced changes in sodium currents are expected to alter excitability substantially and could underlie the molecular pathogenesis of some chronic pain syndromes associated with injury to the axons of spinal sensory neurons.
Figures


Similar articles
-
GDNF and NGF reverse changes in repriming of TTX-sensitive Na(+) currents following axotomy of dorsal root ganglion neurons.J Neurophysiol. 2002 Aug;88(2):650-8. doi: 10.1152/jn.2002.88.2.650. J Neurophysiol. 2002. PMID: 12163518
-
Rescue of alpha-SNS sodium channel expression in small dorsal root ganglion neurons after axotomy by nerve growth factor in vivo.J Neurophysiol. 1998 May;79(5):2668-76. doi: 10.1152/jn.1998.79.5.2668. J Neurophysiol. 1998. PMID: 9582237
-
Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons.Brain Res Mol Brain Res. 1999 Apr 20;67(2):267-82. doi: 10.1016/s0169-328x(99)00070-4. Brain Res Mol Brain Res. 1999. PMID: 10216225
-
Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain.Muscle Nerve. 1999 Sep;22(9):1177-87. doi: 10.1002/(sici)1097-4598(199909)22:9<1177::aid-mus3>3.0.co;2-p. Muscle Nerve. 1999. PMID: 10454712 Review.
-
Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions.J Neurosci. 1998 Mar 15;18(6):2174-87. doi: 10.1523/JNEUROSCI.18-06-02174.1998. J Neurosci. 1998. PMID: 9482802 Free PMC article. Review.
Cited by
-
The Na(V)1.7 sodium channel: from molecule to man.Nat Rev Neurosci. 2013 Jan;14(1):49-62. doi: 10.1038/nrn3404. Epub 2012 Dec 12. Nat Rev Neurosci. 2013. PMID: 23232607 Review.
-
Gastrodin inhibits allodynia and hyperalgesia in painful diabetic neuropathy rats by decreasing excitability of nociceptive primary sensory neurons.PLoS One. 2012;7(6):e39647. doi: 10.1371/journal.pone.0039647. Epub 2012 Jun 25. PLoS One. 2012. PMID: 22761855 Free PMC article.
-
Peripheral drive in Aα/β-fiber neurons is altered in a rat model of osteoarthritis: changes in following frequency and recovery from inactivation.J Pain Res. 2013 Mar 19;6:207-21. doi: 10.2147/JPR.S40445. Print 2013. J Pain Res. 2013. PMID: 23671396 Free PMC article.
-
Modulation of spinal cord synaptic activity by tumor necrosis factor α in a model of peripheral neuropathy.J Neuroinflammation. 2011 Dec 21;8:177. doi: 10.1186/1742-2094-8-177. J Neuroinflammation. 2011. PMID: 22189061 Free PMC article.
-
Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain.J Neurosci. 1999 Oct 1;19(19):8589-96. doi: 10.1523/JNEUROSCI.19-19-08589.1999. J Neurosci. 1999. PMID: 10493758 Free PMC article.
References
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Science. 1996;379:257–262. - PubMed
-
- Appelgren L, Janson M, Nitescu P, Curelaru I. Continuous intracisternal and high cervical intrathecal bupivacaine analgesia in refractory head and neck pain. Anesthesiology. 1996;84:256–272. - PubMed
-
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel α-subunit mRNAs. Mol Brain Res. 1996;43:117–132. - PubMed
-
- Boas RA, Covino BG, Shahnarian A. Analgesic responses to i.v. lignocaine. Br J Anaesth. 1982;54:501–505. - PubMed
-
- Burchiel KJ. Carbamazepine inhibits spontaneous activity in experimental neuromas. Exp Neurol. 1988;102:249–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
