TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death
- PMID: 9133385
- PMCID: PMC6573670
- DOI: 10.1523/JNEUROSCI.17-10-03623.1997
TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death
Abstract
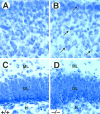
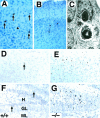
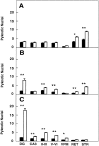
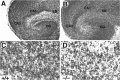
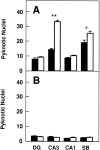
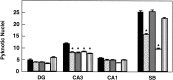
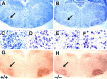
Newborn mice carrying targeted mutations in genes encoding neurotrophins or their signaling Trk receptors display severe neuronal deficits in the peripheral nervous system but not in the CNS. In this study, we show that trkB (-/-) mice have a significant increase in apoptotic cell death in different regions of the brain during early postnatal life. The most affected region in the brain is the dentate gyrus of the hippocampus, although elevated levels of pyknotic nuclei were also detected in cortical layers II and III and V and VI, the striatum, and the thalamus. Furthermore, axotomized hippocampal and motor neurons of trkB (-/-) mice have significantly lower survival rates than those of wild-type littermates. These results suggest that neurotrophin signaling through TrkB receptors plays a role in the survival of CNS neurons during postnatal development. Moreover, they indicate that TrkB receptor signaling protects subpopulations of CNS neurons from injury- and axotomy-induced cell death.
Figures


References
-
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990a;301:325–342. - PubMed
-
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990b;301:365–381. - PubMed
-
- Arenas E, Persson H. Neurotrophin-3 prevents the death of adult central noradrenergic neurons in vivo. Nature. 1994;367:368–371. - PubMed
-
- Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. - PubMed
-
- Bayer SA. Development of the hippocampal region of the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980;190:87–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
