Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-)parabrachio-amygdaloid, in the rat
- PMID: 9133395
- PMCID: PMC6573681
- DOI: 10.1523/JNEUROSCI.17-10-03751.1997
Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-)parabrachio-amygdaloid, in the rat
Abstract
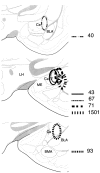
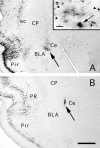
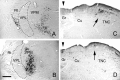
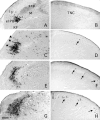
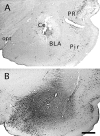
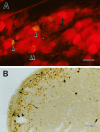
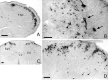
Transneuronal tracing of a nociceptive pathway, the spino-(trigemino)-parabrachio-amygdaloid pathway, was performed using an alpha-herpes virus, the Bartha strain of pseudorabies virus (PRV). Microinjection of PRV into the central nucleus of the amygdala (Ce) resulted in progressive retrograde and transneuronal infection of a multisynaptic circuit involving neurons in the brainstem and spinal cord as detected immunocytochemically. At short survival (26 hr), retrogradely labeled neurons were concentrated in the external lateral nucleus of the parabrachial complex (elPB) but were absent from both the trigeminal nucleus caudalis (TNC) and the spinal cord. At longer survivals (52 hr), labeled cells were present in lamina I of both the TNC and spinal dorsal horn. Retrograde labeling from the Ce with Fluoro-gold demonstrated that elPB neurons have long dendrites extending laterally into the terminal field of spinal and trigeminal afferents, where transneuronal passage of PRV to these afferents could occur. Even longer survivals (76 hr) resulted in a columnar pattern of cell labeling in the TNC and spinal dorsal horn that extended from lamina I into lamina II. At this longest survival, primary sensory neurons became infected. Bilateral excitotoxic lesions of the elPB blocked almost all viral passage from the Ce to superficial laminae of the TNC and spinal dorsal horn. These results demonstrate that nociceptive input to the amygdala is relayed from neurons in lamina I through the elPB. We propose that this modular arrangement of lamina I and II neurons may provide the basis for spinal processing of peripheral input to the amygdala.
Figures





References
-
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdala. In: Aggleton JP, editor. The amygdala. Wiley; New York: 1992. pp. 1–66.
-
- Arvidsson J, Pfaller K. Central projections of C4–C8 dorsal root ganglia in the rat studied by anterograde transport of WGA–HRP. J Comp Neurol. 1990;292:349–362. - PubMed
-
- Bennett GJ, Abdelmoumene M, Hayashi H, Dubner R. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. J Comp Neurol. 1980;194:809–827. - PubMed
-
- Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
