Molecular and physiological diversity of cortical nonpyramidal cells
- PMID: 9133407
- PMCID: PMC6573690
- DOI: 10.1523/JNEUROSCI.17-10-03894.1997
Molecular and physiological diversity of cortical nonpyramidal cells
Abstract
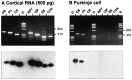
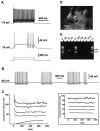
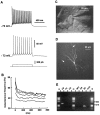
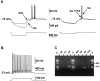
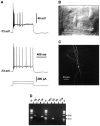
The physiological and molecular features of nonpyramidal cells were investigated in acute slices of sensory-motor cortex using whole-cell recordings combined with single-cell RT-PCR to detect simultaneously the mRNAs of three calcium binding proteins (calbindin D28k, parvalbumin, and calretinin) and four neuropeptides (neuropeptide Y, vasoactive intestinal polypeptide, somatostatin, and cholecystokinin). In the 97 neurons analyzed, all expressed mRNAs of at least one calcium binding protein, and the majority (n = 73) contained mRNAs of at least one neuropeptide. Three groups of nonpyramidal cells were defined according to their firing pattern. (1) Fast spiking cells (n = 34) displayed tonic discharges of fast action potentials with no accommodation. They expressed parvalbumin (n = 30) and/or calbindin (n = 19) mRNAs, and half of them also contained transcripts of at least one of the four neuropeptides. (2) Regular spiking nonpyramidal cells (n = 48) displayed a firing behavior characterized by a marked accommodation and presented a large diversity of expression patterns of the seven biochemical markers. (3) Finally, a small population of vertically oriented bipolar cells, termed irregular spiking cells (n = 15), fired bursts of action potentials at an irregular frequency. They consistently co-expressed calretinin and vasoactive intestinal polypeptide. Additional investigations of these cells showed that they also co-expressed glutamic acid decarboxylase and choline acetyl transferase. Our results indicate that neocortical nonpyramidal neurons display a large diversity in their firing properties and biochemical patterns of co-expression and that both characteristics could be correlated to define discrete subpopulations.
Figures


References
-
- Alcantara S, de Lecea L, Del Rio JA, Ferrer I, Soriano E. Transient colocalization of parvalbumin and calbindin D28K in the postnatal cerebral cortex: evidence for a phenotypic shift in developing nonpyramidal neurons. Eur J Neurosci. 1996;8:1329–1339. - PubMed
-
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. - PubMed
-
- Bochet P, Audinat E, Lambolez B, Crépel F, Rossier J, Iino M, Tsuzuki K, Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–388. - PubMed
-
- Brice A, Berrard S, Raynaud S, Ansieau S, Coppola T, Weber MJ, Mallet J. Complete sequence of a cDNA encoding an active rat choline acetyltransferase: a tool to investigate the plasticity of cholinergic phenotype expression. J Neurosci Res. 1989;23:266–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
