Highly attenuated modified vaccinia virus Ankara (MVA) as an effective recombinant vector: a murine tumor model
- PMID: 9141209
- PMCID: PMC1950787
- DOI: 10.1016/s0264-410x(96)00195-8
Highly attenuated modified vaccinia virus Ankara (MVA) as an effective recombinant vector: a murine tumor model
Abstract
Modified vaccinia virus Ankara (MVA), a highly attenuated strain of vaccinia virus (VV) that is unable to replicate in most mammalian cells, was evaluated as an expression vector for a model tumor associated antigen (TAA) and as a potential anti-cancer vaccine. We employed an experimental murine model in which an adenocarcinoma tumor line, CT26.CL25, was stably transfected with a model TAA, beta-galactosidase (beta-gal). Mice injected intramuscularly with a recombinant MVA (rMVA) expressing beta-gal (MVA-LZ), were protected from a lethal intravenous (i.v.) challenge with CT26.CL25. In addition, splenocytes from mice primed with MVA-LZ were therapeutically effective upon adoptive transfer to mice bearing pulmonary metastases of the CT26.CL25 tumor established 3 days earlier. Most importantly, i.v. inoculation with MVA-LZ resulted in significantly prolonged survival of mice bearing three day old pulmonary metastases. This prolonged survival compared favorably to mice treated with a replication competent recombinant VV expressing beta-gal. These findings indicate that rMVA is an efficacious alternative to the more commonly used replication competent VV for the development of new recombinant anti-cancer vaccines.
Figures
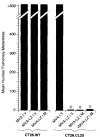




References
-
- Boon T, Gajewski TF, Coulie PG. From defined human tumour antigens to effective immunization? Immunol Today. 1995;16:334–336. - PubMed
-
- Boon T, Cerottini J, Van den Eynde B, van der Bruggen P, Van Pel A. Tumour antigens recognized by T lymphocytes. A Rev Immunol. 1994;12:337–365. - PubMed
-
- Rosenberg SA. The development of new cancer therapies based on the molecular identification of cancer regression antigens. Cancer J. 1995;1:90–l00. - PubMed
-
- Arita I. Virological evidence for the success of the smallpox eradication programme. Nature. 1979;279:293–298. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

