Actions of triethylcholine on neuromuscular transmission. 1961
- PMID: 9142408
- PMCID: PMC3224298
- DOI: 10.1111/j.1476-5381.1997.tb06803.x
Actions of triethylcholine on neuromuscular transmission. 1961
Abstract
The effects of the triethyl analogue of choline (triethyl 2-hydroxyethyl ammonium) on muscular activity have been studied in conscious rabbits, chicks, dogs and a cat. The contractions of the tibialis anticus and soleus muscles of cats under chloralose anaesthesia, and of the tibialis anticus muscle of rabbits under urethane anaesthesia and the isolated diaphragm preparation of the rat were also used. In conscious animals, triethylcholine caused a slowly developing muscular weakness which was more severe after exercise and which resembled the symptoms of myasthenia gravis. In nerve-muscle preparations triethylcholine had a selective action in reducing the contractions of muscles elicited by a high rate of nerve stimulation while leaving unaffected the contractions caused by slower rates of stimulation. During the paralysis of the tibialis muscle of the cat produced by triethylcholine, action potentials recorded from the motor nerve were unaffected and the muscle responded normally to injected acetylcholine and to direct electrical stimulation. The failure of neuromuscular transmission produced by triethylcholine was reversed by injection of choline, but anticholinesterases were ineffective. Choline reduced the toxicity of triethylcholine in mice. It is concluded that triethylcholine produces transmission failure at the neuromuscular junction by interfering with the ability of the nerve endings to synthesize acetylcholine. The possibility that triethylcholine is itself acetylated by the nerve endings and released as an inactive neurohormone is discussed. It was shown that triethylcholine was devoid of depolarizing action and curare-like blocking action. It possesses a transient ganglion blocking action of the tetraethylammonium-type as shown in experiments in which it caused a fall in blood pressure and blocked the response of the nictitating membrane to pre- but not to post-ganglionic stimulation of the cervical sympathetic nerve.
Figures

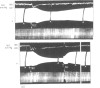

 , the muscles were stimulated directly. The animal had previously recovered from an initial injection of 35 mg/kg of TEC.
, the muscles were stimulated directly. The animal had previously recovered from an initial injection of 35 mg/kg of TEC.





Similar articles
-
The pharmacological actions of polymethylene bistrimethylammonium salts. 1949.Br J Pharmacol. 1997 Feb;120(4 Suppl):60-79; discussion 51-9. doi: 10.1111/j.1476-5381.1997.tb06777.x. Br J Pharmacol. 1997. PMID: 9142396 Free PMC article. No abstract available.
-
Actions of triethylcholine on neuromuscular transmission.Br J Pharmacol Chemother. 1961 Oct;17(2):176-95. doi: 10.1111/j.1476-5381.1961.tb01278.x. Br J Pharmacol Chemother. 1961. PMID: 13872107 Free PMC article.
-
omega-Conotoxin GVIA binds to and blocks rat neuromuscular junction.Neurosci Lett. 1994 Aug 1;176(2):185-8. doi: 10.1016/0304-3940(94)90078-7. Neurosci Lett. 1994. PMID: 7830943
-
Neuromuscular block.Br J Pharmacol. 2006 Jan;147 Suppl 1(Suppl 1):S277-86. doi: 10.1038/sj.bjp.0706404. Br J Pharmacol. 2006. PMID: 16402115 Free PMC article. Review.
-
[Blockade of the ion channels of the skeletal muscle acetylcholine receptor].Eksp Klin Farmakol. 1998 Nov-Dec;61(6):73-5. Eksp Klin Farmakol. 1998. PMID: 9929824 Review. Russian.
References
-
- Appleton HD, Levy BB, Steele JM, Brodie BB. Free choline in plasma. Fed. Proc. 1951;10:157. - PubMed
-
- Augustinsson K-B. Cholinesterases—a study in comparative enzymology. Acta physiol. scand. 1948;15(Suppl. 15):1–182. - PubMed
-
- Bowman WC, Rand MJ. The triethyl analogue of choline and neuromuscular transmission. Lancet. 1961;i:480–481.
Publication types
MeSH terms
Substances
Personal name as subject
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

