Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. 1970
- PMID: 9142414
- PMCID: PMC3224310
- DOI: 10.1111/j.1476-5381.1997.tb06815.x
Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. 1970
Abstract
Stimulation of the vagal non-adrenergic inhibitory innervation caused the release of adenosine and inosine into vascular perfusates from the stomachs of guinea-pigs and toads.
Stimulation of portions of Auerbach's plexus isolated from turkey gizzard caused the release of adenosine triphosphate (ATP), adenosine diphosphate (ADP) and adenosine monophosphate (AMP).
ATP, added to solutions perfused through the toad stomach vasculature, was broken down to adenosine, inosine and adenine.
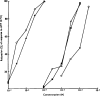
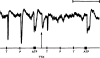
Of a series of purine and pyrimidine derivatives tested for inhibitory activity on the guinea-pig isolated taenia coli, ATP and ADP were the most potent.
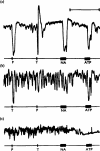
ATP caused inhibition of twelve other gut preparations previously shown to contain non-adrenergic inhibitory nerves. The inhibitory action of ATP was not prevented by tetrodotoxin.
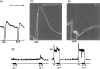
Quinidine antagonized relaxations of the guinea-pig taenia coli caused by catecholamines or adrenergic nerve stimulation. Higher concentrations of quinidine antagonized relaxations caused by ATP or non-adrenergic inhibitory nerve stimulation.
When tachyphylaxis to ATP had been produced in the rabbit ileum, there was a consistent depression of the responses to non-adrenergic inhibitory nerve stimulation but not of responses to adrenergic nerve stimulation.
It is suggested that ATP or a related nucleotide is the transmitter substance released by the non-adrenergic inhibitory innervation of the gut.
Figures

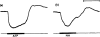
Similar articles
-
Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut.Br J Pharmacol. 1970 Dec;40(4):668-88. doi: 10.1111/j.1476-5381.1970.tb10646.x. Br J Pharmacol. 1970. PMID: 4322041 Free PMC article.
-
Proceedings: A pharmacological analysis of a putative neurotransmitter substance released from the non-adrenergic inhibitory nerve.Jpn J Pharmacol. 1974;24(0):s:133. Jpn J Pharmacol. 1974. PMID: 4151690 No abstract available.
-
[3H]adenosine triphosphate: release during stimulation of enteric nerves.Science. 1971 Jul 23;173(3994):336-8. doi: 10.1126/science.173.3994.336. Science. 1971. PMID: 4327032
-
The non-adrenergic non-cholinergic innervation of the stomach.Arch Int Pharmacodyn Ther. 1986 Apr;280(2 Suppl):84-109. Arch Int Pharmacodyn Ther. 1986. PMID: 2873803 Review. No abstract available.
-
Non-adrenergic non-cholinergic relaxation of the rat stomach.Gen Pharmacol. 1998 Nov;31(5):697-703. doi: 10.1016/s0306-3623(98)00096-2. Gen Pharmacol. 1998. PMID: 9809465 Review.
Cited by
-
Guanosine-Based Nucleotides, the Sons of a Lesser God in the Purinergic Signal Scenario of Excitable Tissues.Int J Mol Sci. 2020 Feb 26;21(5):1591. doi: 10.3390/ijms21051591. Int J Mol Sci. 2020. PMID: 32111063 Free PMC article. Review.
-
Physiological roles and potential therapeutic applications of the P2X7 receptor in inflammation and pain.Molecules. 2013 Sep 5;18(9):10953-72. doi: 10.3390/molecules180910953. Molecules. 2013. PMID: 24013409 Free PMC article. Review.
-
Purinergic receptors in airway hydration.Biochem Pharmacol. 2021 May;187:114387. doi: 10.1016/j.bcp.2020.114387. Epub 2021 Jan 5. Biochem Pharmacol. 2021. PMID: 33358825 Free PMC article. Review.
-
P2 receptors for extracellular nucleotides in the central nervous system: role of P2X7 and P2Y₂ receptor interactions in neuroinflammation.Mol Neurobiol. 2012 Aug;46(1):96-113. doi: 10.1007/s12035-012-8263-z. Epub 2012 Apr 1. Mol Neurobiol. 2012. PMID: 22467178 Free PMC article. Review.
-
Perspectives of purinergic signaling in stem cell differentiation and tissue regeneration.Purinergic Signal. 2012 Sep;8(3):523-37. doi: 10.1007/s11302-011-9282-3. Epub 2011 Dec 6. Purinergic Signal. 2012. PMID: 22143354 Free PMC article. Review.
References
-
- Abood LG, Koketsu K, Miyamoto S. Outflux of various phosphates during membrane depolarization of excitable tissue. Am. J. Physiol. 1962;202:469–474. - PubMed
-
- Axelsson J, Holmberg B. The effects of extracellularly applied ATP and related compounds on electrical and mechanical activity of the smooth muscle taenia coli from the guinea-pig. Acta physiol. scand. 1969;75:149–156. - PubMed
Publication types
MeSH terms
Substances
Personal name as subject
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

