The channel-blocking action of methonium compounds on rat submandibular ganglion cells. 1983
- PMID: 9142425
- PMCID: PMC3224332
- DOI: 10.1111/j.1476-5381.1997.tb06837.x
The channel-blocking action of methonium compounds on rat submandibular ganglion cells. 1983
Abstract
The effects of drugs of the polymethylene bis-trimethylammonium (methonium) series on the characteristics of the synaptic currents (e.s.cs) recorded from voltage-clamped rat submandibular ganglion cells have been studied. The drugs studied were from C4 to C10 (decamethonium).
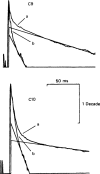
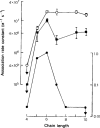
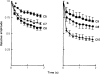
All of the drugs except C4 shortened the initial decay phase of the e.s.c.; C9 and C10 produced an additional slowly decaying component. These effects were interpreted in terms of an open channel block mechanism, the calculated rate constants for association with the open channel at − 80 mV being fairly similar (5.9 × 106 to 18.1 × 106M−1 s−1) for all of the compounds except C4, which had no effect on the e.s.c. decay.
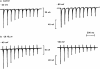
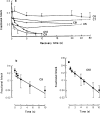
All of the compounds produced use-dependent block when tested with short trains of stimuli at 10 Hz, or with trains of ionophoretic pulses of acetylcholine, consistent with their channel blocking property. Tubocurarine had a similar effect, but not trimetaphan or mecamylamine.
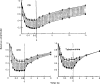
Recovery from use-dependent block with short chain methonium compounds, up to C8, was very slow in the absence of agonist, being incomplete even after several minutes. With C9 or C10 or tubocurarine, recovery from use-dependent block was complete within a few seconds. With C6 recovery in the absence of agonist was unaffected by membrane potential, but could be accelerated by applying acetylcholine with the cell depolarized to − 40 mV. This persistent block was ascribed to the ability of the blocking molecule to become trapped by closure of the channel. With C9 and C10 it is assumed that their presence inhibits channel closure, so they can escape without the help of agonist.
When use-dependent block is avoided by leaving the ganglion unstimulated during equilibration with the blocking drug, the first e.s.c. elicited shows no appreciable reduction of amplitude, though with C6, C7 or C8 subsequent responses elicited at 0.1 Hz become progressively more blocked.
Even at 1 M
m , C6 does not prevent acetylcholine from opening ionic channels.It is concluded that all of the effects on e.s.c. amplitude can be interpreted in terms of channel block, there being no evidence of any receptor blocking action.
Figures

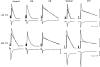



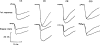


Similar articles
-
The channel-blocking action of methonium compounds on rat submandibular ganglion cells.Br J Pharmacol. 1984 Jul;82(3):623-42. doi: 10.1111/j.1476-5381.1984.tb10801.x. Br J Pharmacol. 1984. PMID: 6146366 Free PMC article.
-
The rational use of neuromuscular blocking agents: the role of pancuronium.Drugs. 1972;4(3):153-62. doi: 10.2165/00003495-197204030-00001. Drugs. 1972. PMID: 4118863 No abstract available.
-
[Ganglionic-blockading action of bis-ammonium compounds].Neirofiziologiia. 1984;16(1):54-61. Neirofiziologiia. 1984. PMID: 6144051 Russian.
-
Drugs and ionic channels: mechanisms and implications.Postgrad Med J. 1981;57 Suppl 1:89-97. Postgrad Med J. 1981. PMID: 6117850 Review.
-
[VASOMOTOR EFFECTS OF GANGLIOPLEGIC AGENTS].Arch Ital Sci Farmacol. 1963 Apr;13:97-117. Arch Ital Sci Farmacol. 1963. PMID: 14245141 Review. Italian. No abstract available.
References
-
- Adams PR. Acetylcholine receptor kinetics. J. memb. Biol. 1981;58:161–174. - PubMed
Publication types
MeSH terms
Substances
Personal name as subject
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

