Crystal structure of glutamate-1-semialdehyde aminomutase: an alpha2-dimeric vitamin B6-dependent enzyme with asymmetry in structure and active site reactivity
- PMID: 9144156
- PMCID: PMC24597
- DOI: 10.1073/pnas.94.10.4866
Crystal structure of glutamate-1-semialdehyde aminomutase: an alpha2-dimeric vitamin B6-dependent enzyme with asymmetry in structure and active site reactivity
Abstract
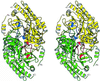
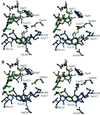
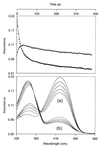
The three-dimensional structure of glutamate-1-semialdehyde aminomutase (EC 5.4.3.8), an alpha2-dimeric enzyme from Synechococcus, has been determined by x-ray crystallography using heavy atom derivative phasing. The structure, refined at 2.4-A resolution to an R-factor of 18.7% and good stereochemistry, explains many of the enzyme's unusual specificity and functional properties. The overall fold is that of aspartate aminotransferase and related B6 enzymes, but it also has specific features. The structure of the complex with gabaculine, a substrate analogue, shows unexpectedly that the substrate binding site involves residues from the N-terminal domain of the molecule, notably Arg-32. Glu-406 is suitably positioned to repel alpha-carboxylic acids, thereby suggesting a basis for the enzyme's reaction specificity. The subunits show asymmetry in cofactor binding and in the mobilities of the residues 153-181. In the unliganded enzyme, one subunit has the cofactor bound as an aldimine of pyridoxal phosphate with Lys-273 and, in this subunit, residues 153-181 are disordered. In the other subunit in which the cofactor is not covalently bound, residues 153-181 are well defined. Consistent with the crystallographically demonstrated asymmetry, a form of the enzyme in which both subunits have pyridoxal phosphate bound to Lys-273 through a Schiff base showed biphasic reduction by borohydride in solution. Analysis of absorption spectra during reduction provided evidence of communication between the subunits. The crystal structure of the reduced form of the enzyme shows that, despite identical cofactor binding in each monomer, the structural asymmetry at residues 153-181 remains.
Figures
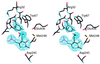
References
-
- Alexander F W, Sandmeier E, Mehta P K, Christen P. Eur J Biochem. 1994;219:953–960. - PubMed
-
- Jordan P M, Shemin D. In: The Enzymes. 3rd Ed. Boyer P D, editor. Vol. 7. New York: Academic; 1972. pp. 339–356.
-
- Kannangara G, Gough S P, Bryant P, Hoober J K, Kahn A, von Wettstein D. Trends Biochem Sci. 1988;13:139–143. - PubMed
-
- Grimm B, Bull A, Breu V. Mol Gen Genet. 1991;225:1–10. - PubMed
-
- Mehta P K, Christen P. Biochem Biophys Res Commun. 1994;198:138–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

