Karyopherin beta2 mediates nuclear import of a mRNA binding protein
- PMID: 9144189
- PMCID: PMC24630
- DOI: 10.1073/pnas.94.10.5055
Karyopherin beta2 mediates nuclear import of a mRNA binding protein
Abstract
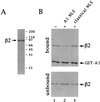
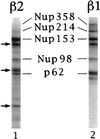
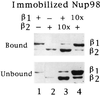
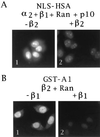
We have cloned and sequenced cDNA for human karyopherin beta2, also known as transportin. In a solution binding assay, recombinant beta2 bound directly to recombinant nuclear mRNA-binding protein A1. Binding was inhibited by a peptide representing A1's previously characterized M9 nuclear localization sequence (NLS), but not by a peptide representing a classical NLS. As previously shown for karyopherin beta1, karyopherin beta2 bound to several nucleoporins containing characteristic peptide repeat motifs. In a solution binding assay, both beta1 and beta2 competed with each other for binding to immobilized repeat nucleoporin Nup98. In digitonin-permeabilized cells, beta2 was able to dock A1 at the nuclear rim and to import it into the nucleoplasm. At low concentrations of beta2, there was no stimulation of import by the exogenous addition of the GTPase Ran. However, at higher concentrations of beta2 there was marked stimulation of import by Ran. Import was inhibited by the nonhydrolyzable GTP analog guanylyl imidodiphosphate by a Ran mutant that is unable to hydrolyze GTP and also by wheat germ agglutinin. Consistent with the solution binding results, karyopherin beta2 inhibited karyopherin alpha/beta1-mediated import of a classical NLS containing substrate and, vice versa, beta1 inhibited beta2-mediated import of A1 substrate, suggesting that the two import pathways merge at the level of docking of beta1 and beta2 to repeat nucleoporins.
Figures


References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

