The origin and evolution of animal appendages
- PMID: 9144208
- PMCID: PMC24649
- DOI: 10.1073/pnas.94.10.5162
The origin and evolution of animal appendages
Abstract
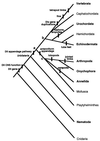
Animals have evolved diverse appendages adapted for locomotion, feeding and other functions. The genetics underlying appendage formation are best understood in insects and vertebrates. The expression of the Distal-less (Dll) homeoprotein during arthropod limb outgrowth and of Dll orthologs (Dlx) in fish fin and tetrapod limb buds led us to examine whether expression of this regulatory gene may be a general feature of appendage formation in protostomes and deuterostomes. We find that Dll is expressed along the proximodistal axis of developing polychaete annelid parapodia, onychophoran lobopodia, ascidian ampullae, and even echinoderm tube feet. Dll/Dlx expression in such diverse appendages in these six coelomate phyla could be convergent, but this would have required the independent co-option of Dll/Dlx several times in evolution. It appears more likely that ectodermal Dll/Dlx expression along proximodistal axes originated once in a common ancestor and has been used subsequently to pattern body wall outgrowths in a variety of organisms. We suggest that this pre-Cambrian ancestor of most protostomes and the deuterostomes possessed elements of the genetic machinery for and may have even borne appendages.
Figures



References
-
- Wang B B, Muller-Immergluck M M, Austin J, Robinson N T, Chisholm A, Kenyon C. Cell. 1993;74:29–42. - PubMed
-
- Cohen S M. Nature (London) 1990;343:173–177. - PubMed
-
- Panganiban G, Nagy L, Carroll S B. Curr Biol. 1994;4:671–675. - PubMed
-
- Panganiban G, Sebring A, Nagy L, Carroll S B. Science. 1995;270:1363–1366. - PubMed
-
- Popadic A, Rusch D, Peterson M, Rogers B T, Kaufman T C. Nature (London) 1996;380:395.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

