Functional domains are specified to single-cell resolution in a Drosophila epithelium
- PMID: 9144216
- PMCID: PMC24657
- DOI: 10.1073/pnas.94.10.5207
Functional domains are specified to single-cell resolution in a Drosophila epithelium
Abstract
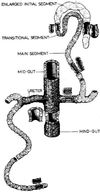
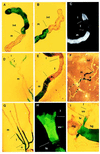
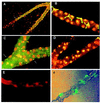
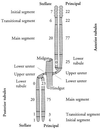
Specification of pattern is fundamental to the development of a multicellular organism. The Malpighian (renal) tubule of Drosophila melanogaster is a simple epithelium that proliferates under the direction of a single tip cell into three morphologically distinct domains. However, systematic analysis of a panel of over 700 P[GAL4] enhancer trap lines reveals unexpected richness for such an apparently simple tissue. Using numerical analysis, it was possible formally to reconcile apparently similar or complementary expression domains and thus to define at least five genetically defined domains and multiple cell types. Remarkably, the positions of domain boundaries and the numbers of both principal and secondary ("stellate") cell types within each domain are reproducible to near single-cell precision between individual animals. Domains of physiological function were also mapped using transport or expression assays. Invariably, they respect the boundaries defined by enhancer activity. These genetic domains can also be visualized in vivo, both in transgenic and wild-type flies, providing an "identified cell" system for epithelial physiology. Building upon recent advances in Drosophila Malpighian tubule physiology, the present study confirms this tissue as a singular model for integrative physiology.
Figures



References
-
- Bertram M J, Akerkar G A, Ard R L, Gonzalez C, Wolfner M F. Mech Dev. 1992;38:33–40. - PubMed
-
- Hartenstein V, Jan Y N. Roux’s Arch Dev Biol. 1992;201:194–220. - PubMed
-
- Riesgo-Escovar J, Woodard C, Gaines P, Carlson J. J Neurobiol. 1992;23:947–964. - PubMed
-
- Doe C Q, ChuLaGraff Q, Wright D M, Scott M P. Cell. 1991;65:451–464. - PubMed
-
- Nose A, Mahajan V B, Goodman C S. Cell. 1992;70:553–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

