Acute leukemia with promyelocytic features in PML/RARalpha transgenic mice
- PMID: 9144232
- PMCID: PMC24673
- DOI: 10.1073/pnas.94.10.5302
Acute leukemia with promyelocytic features in PML/RARalpha transgenic mice
Abstract
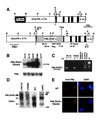
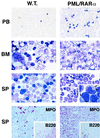
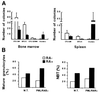
Acute promyelocytic leukemia (APL) is associated with reciprocal chromosomal translocations involving the retinoic acid receptor alpha (RARalpha) locus on chromosome 17. In the majority of cases, RARalpha translocates and fuses with the promyelocytic leukemia (PML) gene located on chromosome 15. The resulting fusion genes encode the two structurally unique PML/RARalpha and RARalpha/PML fusion proteins as well as aberrant PML gene products, the respective pathogenetic roles of which have not been elucidated. We have generated transgenic mice in which the PML/RARalpha fusion protein is specifically expressed in the myeloid-promyelocytic lineage. During their first year of life, all the PML/RARalpha transgenic mice have an abnormal hematopoiesis that can best be described as a myeloproliferative disorder. Between 12 and 14 months of age, 10% of them develop a form of acute leukemia with a differentiation block at the promyelocytic stage that closely mimics human APL even in its response to retinoic acid. Our results are conclusive in vivo evidence that PML/RARalpha plays a crucial role in the pathogenesis of APL.
Figures

References
-
- Bennett J M, Catovsky D, Daniel D, Flandrin G, Galton D A G, Gralnick H R, Sultan C. Br J Haematol. 1976;33:451–458. - PubMed
-
- Pandolfi P P. Haematologica. 1996;81:472–482. - PubMed
-
- Grignani F, Fagioli M, Alcalay M, Longo L, Pandolfi P P, Donti E, Biondi A, Lo Coco F, Pelicci P G. Blood. 1994;83:10–25. - PubMed
-
- Warrell R P, Jr, de Thé H, Wang Z Y, Degos L. N Engl J Med. 1993;329:177–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

