Slow skeletal troponin I gene transfer, expression, and myofilament incorporation enhances adult cardiac myocyte contractile function
- PMID: 9144257
- PMCID: PMC24698
- DOI: 10.1073/pnas.94.10.5444
Slow skeletal troponin I gene transfer, expression, and myofilament incorporation enhances adult cardiac myocyte contractile function
Abstract
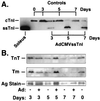
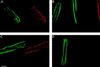
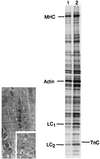
The functional significance of the developmental transition from slow skeletal troponin I (ssTnI) to cardiac TnI (cTnI) isoform expression in cardiac myocytes remains unclear. We show here the effects of adenovirus-mediated ssTnI gene transfer on myofilament structure and function in adult cardiac myocytes in primary culture. Gene transfer resulted in the rapid, uniform, and nearly complete replacement of endogenous cTnI with the ssTnI isoform with no detected changes in sarcomeric ultrastructure, or in the isoforms and stoichiometry of other myofilament proteins compared with control myocytes over 7 days in primary culture. In functional studies on permeabilized single cardiac myocytes, the threshold for Ca2+-activated contraction was significantly lowered in adult cardiac myocytes expressing ssTnI relative to control values. The tension-Ca2+ relationship was unchanged from controls in primary cultures of cardiac myocytes treated with adenovirus containing the adult cardiac troponin T (TnT) or cTnI cDNAs. These results indicate that changes in Ca2+ activation of tension in ssTnI-expressing cardiac myocytes were isoform-specific, and not due to nonspecific functional changes resulting from overexpression of a myofilament protein. Further, Ca2+-activated tension development was enhanced in cardiac myocytes expressing ssTnI compared with control values under conditions mimicking the acidosis found during myocardial ischemia. These results show that ssTnI enhances contractile sensitivity to Ca2+ activation under physiological and acidic pH conditions in adult rat cardiac myocytes, and demonstrate the utility of adenovirus vectors for rapid and efficient genetic modification of the cardiac myofilament for structure/function studies in cardiac myocytes.
Figures
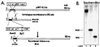
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

