Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells
- PMID: 9151667
- PMCID: PMC2139876
- DOI: 10.1083/jcb.137.3.595
Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells
Abstract
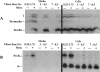
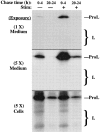
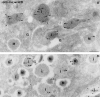
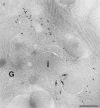
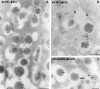
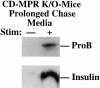
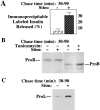
In cells specialized for secretory granule exocytosis, lysosomal hydrolases may enter the regulated secretory pathway. Using mouse pancreatic islets and the INS-1 beta-cell line as models, we have compared the itineraries of procathepsins L and B, two closely related members of the papain superfamily known to exhibit low and high affinity for mannose-6-phosphate receptors (MPRs), respectively. Interestingly, shortly after pulse labeling INS cells, a substantial fraction of both proenzymes exhibit regulated exocytosis. After several hours, much procathepsin L remains as precursor in a compartment that persists in its ability to undergo regulated exocytosis in parallel with insulin, while procathepsin B is efficiently converted to the mature form and can no longer be secreted. However, in islets from transgenic mice devoid of cation-dependent MPRs, the modest fraction of procathepsin B normally remaining within mature secretory granules is increased approximately fourfold. In normal mouse islets, immunoelectron microscopy established that both cathepsins are present in immature beta-granules, while immunolabeling for cathepsin L, but not B, persists in mature beta-granules. By contrast, in islets from normal male Sprague-Dawley rats, much of the proenzyme sorting appears to occur earlier, significantly diminishing the stimulus-dependent release of procathepsin B. Evidently, in the context of different systems, MPR-mediated sorting of lysosomal proenzymes occurs to a variable extent within the trans-Golgi network and is continued, as needed, within immature secretory granules. Lysosomal proenzymes that fail to be sorted at both sites remain as residents of mature secretory granules.
Figures