An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation
- PMID: 9151685
- PMCID: PMC2139836
- DOI: 10.1083/jcb.137.4.825
An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation
Abstract
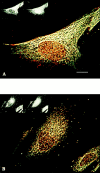
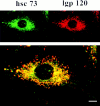
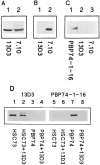
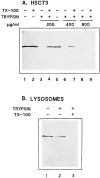
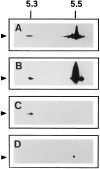
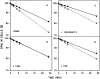
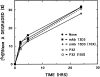
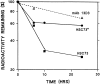
Previous studies have implicated the heat shock cognate (hsc) protein of 73 kD (hsc73) in stimulating a lysosomal pathway of proteolysis that is selective for particular cytosolic proteins. This pathway is activated by serum deprivation in confluent cultured human fibroblasts. We now show, using indirect immunofluorescence and laser scanning confocal microscopy, that a heat shock protein (hsp) of the 70-kD family (hsp70) is associated with lysosomes (ly-hsc73). An mAb designated 13D3 specifically recognizes hsc73, and this antibody colocalizes with an antibody to lgp120, a lysosomal marker protein. Most, but not all, lysosomes contain ly-hsc73, and the morphological appearance of these organelles dramatically changes in response to serum withdrawal; the punctate lysosomes fuse to form tubules. Based on susceptibility to digestion by trypsin and by immunoblot analysis after two-dimensional electrophoresis of isolated lysosomes and isolated lysosomal membranes, most ly-hsc73 is within the lysosomal lumen. We determined the functional importance of the ly-hsc73 by radiolabeling cellular proteins with [3H]leucine and then allowing cells to endocytose excess mAb 13D3 before measuring protein degradation in the presence and absence of serum. The increased protein degradation in response to serum deprivation was completely inhibited by endocytosed mAb 13D3, while protein degradation in cells maintained in the presence of serum was unaffected. The intralysosomal digestion of endocytosed [3H]RNase A was not affected by the endocytosed mAb 13D3. These results suggest that ly-hsc73 is required for a step in the degradative pathway before protein digestion within lysosomes, most likely for the import of substrate proteins.
Figures

References
-
- Amenta J, Brocher S. Mechanism of protein turnover in cultured cells. Life Sci. 1981;28:1195–1208. - PubMed
-
- Aniento F, Roche E, Cuervo AM, Knecht E. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J Biol Chem. 1993;268:10463–10470. - PubMed
-
- Bhattacharyya T, Karnezis AN, Murphy SP, Hoang T, Freeman BC, Phillips B, Morimoto RI. Cloning and subcellular localization of human mitochondrial hsp70. J Biol Chem. 1995;270:1705–1710. - PubMed
-
- Beckman RP, Mizzen LA, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science (Wash DC) 1990;248:850–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

