Characterization of the adaptor-related protein complex, AP-3
- PMID: 9151686
- PMCID: PMC2139840
- DOI: 10.1083/jcb.137.4.835
Characterization of the adaptor-related protein complex, AP-3
Abstract
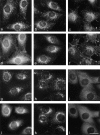
We have recently shown that two proteins related to two of the adaptor subunits of clathrincoated vesicles, p47 (mu3) and beta-NAP (beta3B), are part of an adaptor-like complex not associated with clathrin (Simpson, F., N.A. Bright, M.A. West, L.S. Newman, R.B. Darnell, and M.S. Robinson, 1996. J. Cell Biol. 133:749-760). In the present study we have searched the EST database and have identified, cloned, and sequenced a ubiquitously expressed homologue of beta-NAP, beta3A, as well as homologues of the alpha/gamma and sigma adaptor subunits, delta and sigma3, which are also ubiquitously expressed. Antibodies raised against recombinant delta and sigma3 show that they are the other two subunits of the adaptor-like complex. We are calling this complex AP-3, a name that has also been used for the neuronalspecific phosphoprotein AP180, but we feel that it is a more appropriate designation for an adaptor-related heterotetramer. Immunofluorescence using anti-delta antibodies reveals that the AP-3 complex is associated with the Golgi region of the cell as well as with more peripheral structures. These peripheral structures show only limited colocalization with endosomal markers and may correspond to a postTGN biosynthetic compartment. The delta subunit is closely related to the protein product of the Drosophila garnet gene, which when mutated results in reduced pigmentation of the eyes and other tissues. Because pigment granules are believed to be similar to lysosomes, this suggests either that the AP-3 complex may be directly involved in trafficking to lysosomes or alternatively that it may be involved in another pathway, but that missorting in that pathway may indirectly lead to defects in pigment granules.
Figures







References
-
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SA, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophilagene involved in endocytosis. Nature (Lond) 1991;351:583–586. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

