Cloning and functional characterization of Roaz, a zinc finger protein that interacts with O/E-1 to regulate gene expression: implications for olfactory neuronal development
- PMID: 9151733
- PMCID: PMC6573535
- DOI: 10.1523/JNEUROSCI.17-11-04159.1997
Cloning and functional characterization of Roaz, a zinc finger protein that interacts with O/E-1 to regulate gene expression: implications for olfactory neuronal development
Abstract
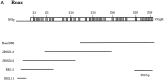
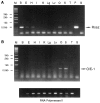
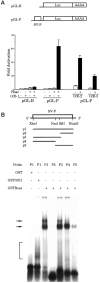
We have identified a protein, Rat O/E-1-associated zinc finger protein (Roaz), that plays a role in regulating the temporal and spatial pattern of olfactory neuronal-specific gene expression. This protein functions by interacting with the olfactory factor O/E-1 and modulating its transcriptional activity. Roaz, isolated via a yeast two-hybrid screen, encoded a protein containing 29 C2H2 zinc fingers of the TFIIIA type. The Roaz mRNA was found in brain, eye, olfactory epithelium, spleen, and heart. In situ hybridization data indicated that Roaz was expressed in the basal layer, consisting of neural precursor cells and immature sensory neurons of the olfactory epithelium, but not in the mature receptor cells. We showed that the Roaz protein bound specifically to O/E-1 by using the yeast two-hybrid system. The two proteins formed a stable complex in coimmunoprecipitation and in vitro binding assays. Introduction of Roaz and O/E-1 into cells containing an olfactory promoter-driven luciferase reporter demonstrated that Roaz abolished O/E-1-mediated transcriptional activation. We propose that the function of Roaz is to modulate negatively the transactivational activity of O/E-1 and to act as a switch protein in the coordination of olfactory sensory neuron differentiation.
Figures






References
-
- Anthony-Cahill SJ, Benfield PA, Fairman R, Wasserman ZR, Brenner SL, Stafford WD, Altenbach C, Hubbell WL, DeGrado WF. Molecular characterization of helix-loop-helix peptides. Science. 1992;255:979–983. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Wiley; New York: 1995.
-
- Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. - PubMed
-
- Bakalyar HA, Reed RR. The second messenger cascade in olfactory receptor neurons. Curr Opin Neurobiol. 1991;1:204–208. - PubMed
-
- Baylies MK, Bate M. twist: a myogenic switch in Drosophila. Science. 1996;272:1481–1484. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
