Upregulation of the endosomal-lysosomal pathway in the trembler-J neuropathy
- PMID: 9151736
- PMCID: PMC6573524
- DOI: 10.1523/JNEUROSCI.17-11-04190.1997
Upregulation of the endosomal-lysosomal pathway in the trembler-J neuropathy
Abstract
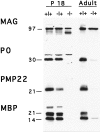
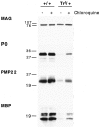
A nonconservative leucine to proline mutation in peripheral myelin protein 22 (PMP22) causes the Trembler-J (TrJ) neuropathy in mice and humans. The expression levels and localization of the PMP22 protein in the TrJ mouse have not been previously determined. The aim of our studies was to reevaluate the extent of myelin deficit in genotyped heterozygous and homozygous animals and to examine how the TrJ mutation alters the normal in vivo post-translational processing of PMP22. Morphological studies show evidence for primary dysmyelination and myelin instability in affected animals. As expected, Western blot analysis indicates that in adult heterozygous TrJ animals, the level of PMP22 is markedly decreased, similar to myelin basic protein and protein zero, whereas myelin-associated glycoprotein is largely unaffected. The decrease in myelin protein expression is associated with an increase in lysosomal biogenesis, suggestive of augmented endocytosis or autophagy. Double-immunolabeling experiments show the accumulation of PMP22 in endosomal/lysosomal structures of TrJ Schwann cells, and chloroquine treatment of nerve segments indicates that the degradation of protein zero, PMP22, and myelin basic protein is augmented in TrJ nerves. These studies suggest that the TrJ mutation alters myelin stability and that the mutant protein is likely degraded via the lysosomal pathway.
Figures






Similar articles
-
Transport of Trembler-J mutant peripheral myelin protein 22 is blocked in the intermediate compartment and affects the transport of the wild-type protein by direct interaction.J Neurosci. 1999 Mar 15;19(6):2027-36. doi: 10.1523/JNEUROSCI.19-06-02027.1999. J Neurosci. 1999. PMID: 10066256 Free PMC article.
-
Overloaded endoplasmic reticulum-Golgi compartments, a possible pathomechanism of peripheral neuropathies caused by mutations of the peripheral myelin protein PMP22.J Neurosci. 1998 Jan 15;18(2):731-40. doi: 10.1523/JNEUROSCI.18-02-00731.1998. J Neurosci. 1998. PMID: 9425015 Free PMC article.
-
Emerging role for autophagy in the removal of aggresomes in Schwann cells.J Neurosci. 2003 Nov 19;23(33):10672-80. doi: 10.1523/JNEUROSCI.23-33-10672.2003. J Neurosci. 2003. PMID: 14627652 Free PMC article.
-
Trembler as a mouse model of CMT1A?Ann N Y Acad Sci. 1999 Sep 14;883:262-72. Ann N Y Acad Sci. 1999. PMID: 10586251 Review.
-
Regulation of myelin-specific gene expression. Relevance to CMT1.Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
Cited by
-
Association of calnexin with mutant peripheral myelin protein-22 ex vivo: a basis for "gain-of-function" ER diseases.Proc Natl Acad Sci U S A. 2002 Jul 23;99(15):9852-7. doi: 10.1073/pnas.152621799. Epub 2002 Jul 15. Proc Natl Acad Sci U S A. 2002. PMID: 12119418 Free PMC article.
-
Reversible folding of human peripheral myelin protein 22, a tetraspan membrane protein.Biochemistry. 2013 May 14;52(19):3229-41. doi: 10.1021/bi301635f. Epub 2013 May 2. Biochemistry. 2013. PMID: 23639031 Free PMC article.
-
Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells into Schwann-Like Cells Improves Neuromuscular Function in a Mouse Model of Charcot-Marie-Tooth Disease Type 1A.Int J Mol Sci. 2018 Aug 14;19(8):2393. doi: 10.3390/ijms19082393. Int J Mol Sci. 2018. PMID: 30110925 Free PMC article.
-
Rer1 and calnexin regulate endoplasmic reticulum retention of a peripheral myelin protein 22 mutant that causes type 1A Charcot-Marie-Tooth disease.Sci Rep. 2014 Nov 11;4:6992. doi: 10.1038/srep06992. Sci Rep. 2014. PMID: 25385046 Free PMC article.
-
Caveats in the Established Understanding of CMT1A.Ann Clin Transl Neurol. 2017 Jun 15;4(8):601-607. doi: 10.1002/acn3.432. eCollection 2017 Aug. Ann Clin Transl Neurol. 2017. PMID: 28812050 Free PMC article.
References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in Pmp22-deficient mice. Nat Genet. 1995;11:274–280. - PubMed
-
- Amara JF, Cheng SH, Smith AE. Intracellular protein trafficking defects in human disease. Trends Cell Biol. 1992;2:145–149. - PubMed
-
- Ayers MM, Anderson RM. Development of onion bulb neuropathy in the trembler mouse. Acta Neuropathol. 1976;36:137–152. - PubMed
-
- Bascles L, Bonnet J, Garbay B. Expression of the PMP22 gene in trembler mutant mice: comparison with other myelin protein genes. Dev Neurosci. 1992;14:336–341. - PubMed
-
- Bonifacio JS, Lippincott-Schwartz J. Degradation of proteins within the endoplasmic reticulum. Curr Opin Cell Biol. 1991;3:592–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
