Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals
- PMID: 9151738
- PMCID: PMC6573527
- DOI: 10.1523/JNEUROSCI.17-11-04212.1997
Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals
Abstract
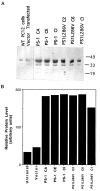
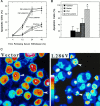
Most autosomal dominant inherited forms of early onset Alzheimer's disease (AD) are caused by mutations in the presenilin-1 (PS-1) gene on chromosome 14. PS-1 is an integral membrane protein with six to nine membrane-spanning domains and is expressed in neurons throughout the brain wherein it is localized mainly in endoplasmic reticulum (ER). The mechanism or mechanisms whereby PS-1 mutations promote neuron degeneration in AD are unknown. Recent findings suggest links among deposition of amyloid beta-peptide (Abeta), oxidative stress, disruption of ion homeostasis, and an apoptotic form of neuron death in AD. We now report that expression of the human PS-1 L286V mutation in PC12 cells increases their susceptibility to apoptosis induced by trophic factor withdrawal and Abeta. Increases in oxidative stress and intracellular calcium levels induced by the apoptotic stimuli were exacerbated greatly in cells expressing the PS-1 mutation, as compared with control cell lines and lines overexpressing wild-type PS-1. The antiapoptotic gene product Bcl-2 prevented apoptosis after NGF withdrawal from differentiated PC12 cells expressing mutant PS-1. Elevations of [Ca2+]i in response to thapsigargin, an inhibitor of the ER Ca2+-ATPase, were increased in cells expressing mutant PS-1, and this adverse effect was abolished in cells expressing Bcl-2. Antioxidants and blockers of calcium influx and release from ER protected cells against the adverse consequences of the PS-1 mutation. By perturbing cellular calcium regulation and promoting oxidative stress, PS-1 mutations may sensitize neurons to apoptotic death in AD.
Figures






References
-
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β-protein toxicity. Cell. 1994;77:817–827. - PubMed
-
- Benzi G, Moretti A. Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol Aging. 1995;16:661–674. - PubMed
-
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada C-M, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin-1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
