Selective expression of dopamine D3 receptor mRNA in proliferative zones during embryonic development of the rat brain
- PMID: 9151745
- PMCID: PMC6573556
- DOI: 10.1523/JNEUROSCI.17-11-04282.1997
Selective expression of dopamine D3 receptor mRNA in proliferative zones during embryonic development of the rat brain
Abstract
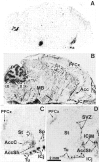
We studied by in situ hybridization histochemistry the expression of D3 receptor (D3R) mRNA at various stages of rat brain development. The first expression of D3R mRNA was detected at embryonic day 14 (E14) in the striatal and rhinencephalic neuroepithelia and throughout the tectal neuroepithelium. From E16 to E19 D3R mRNA expression extended along a rostrocaudal axis to additional proliferative ventricular zones of the basal forebrain, including the neuroepithelia of the olfactory bulb, nucleus accumbens, septum, and amygdala, whereas D1 and D2 receptor (D1R and D2R) mRNAs were expressed predominantly by migrating neuroblasts and/or differentiating striatal neurons. Only a few neuroblasts, migrating in the lateral cortical stream or developing as cerebellar Purkinje cells, expressed D3R mRNA from E18. At birth D3R expression mRNA appeared in differentiating neuronal fields of the nucleus accumbens and medial mamillary body primordia and on P5 reached a distribution similar to that found in adult. In addition, a transient upregulation was detected on P5 in the medial mamillary bodies, parietofrontal cortex, and olfactory tubercle. In the adult brain D3R gene expression continued in the striatal proliferative subventricular zone. The late expression D3R mRNA in neurons, after achievement of dopamine innervation, supports the existence of a regulating factor released from dopamine neurons, as suggested by denervation studies in the adult. The sustained and abundant D3R gene expression, predominantly in germinative neuroepithelial zones actively involved in neurogenesis of most basal forebrain structures, supports the hypothesis of a neurogenetic but minor morphogenetic modulatory role for the D3R during CNS development.
Figures








References
-
- Altman J, Bayer SA. Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J Comp Neurol. 1985;231:42–65. - PubMed
-
- Altman J, Bayer SA. Vertical compartmentation and cellular transformations in the germinal matrices of the embryonic rat cerebral cortex. Exp Neurol. 1990;107:23–35. - PubMed
-
- Arenander AT, De Vellis J. Development of the nervous system. In: Siegel GJ, editor. Basic neurochemistry: molecular, cellular, and medical aspects. Raven; New York: 1994. pp. 573–606.
-
- Bayer SA, Altman J. Cell migration in the developing neocortex. In: Bayer SA, Altman J, editors. Neocortical development. Raven; New York: 1991. pp. 116–127.
-
- Bayer SA, Altman J. Principles of neurogenesis, neuronal migration, and neuronal circuit formation. In: Paxinos G, editor. The rat nervous system. Academic; New York: 1995. pp. 1079–1098.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
