Transient changes in flocculonodular lobe protein kinase C expression during vestibular compensation
- PMID: 9151753
- PMCID: PMC6573528
- DOI: 10.1523/JNEUROSCI.17-11-04367.1997
Transient changes in flocculonodular lobe protein kinase C expression during vestibular compensation
Abstract
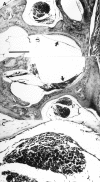
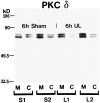
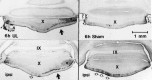
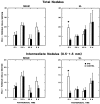
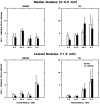
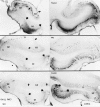
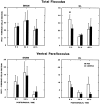
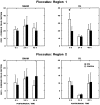
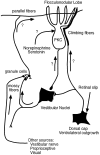
Protein kinase C (PKC) is a family of intracellular signal transduction enzymes, comprising isoforms that vary in sensitivity to calcium, arachidonic acid, and diacylglycerol. PKC isoforms alpha, gamma, and delta are expressed by cerebellar Purkinje cells and neurons in the cerebellar nuclei and vestibular nuclei of the Long-Evans rat. In control rats, these PKCs are distributed symmetrically in the flocculonodular-lobe Purkinje cells. Behavioral recovery from vestibular dysfunction produced by unilateral labyrinthectomy (UL) is accompanied by asymmetric expression of PKC isoforms in these regions within 6 hr after UL. These expression changes were localized within parasagittal regions of the flocculus and nodulus. The distribution of PKCalpha, -gamma, and -delta were identical, suggesting that they are coregulated in cerebellar Purkinje cells during this early compensatory period. The pattern of Purkinje cell PKC expression returned to the control, symmetric distribution within 24 hr after UL. It is hypothesized that these regional changes in Purkinje cell PKC expression are an early intracellular signal contributing to vestibular compensation. In particular, regulation of PKC expression may contribute to changes in the efficacy of cerebellar synaptic plasticity during the acute post-UL period.
Figures



References
-
- Azzi A, Boscoboinik D, Hensey C. The protein kinase C family. Eur J Biochem. 1992;208:547–557. - PubMed
-
- Balaban CD. Olivovestibular and cerebellovestibular connections in albino rabbits. Neuroscience. 1984;12:129–149. - PubMed
-
- Balaban CD. Distribution of inferior olivary projections to the vestibular nuclei of albino rabbits. Neuroscience. 1988;24:119–134. - PubMed
-
- Balaban CD, Henry RT. Zonal organization of olivo-nodulus projections in albino rabbits. Neurosci Res. 1988;5:409–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
