Separation of gating properties from permeation and block in mslo large conductance Ca-activated K+ channels
- PMID: 9154909
- PMCID: PMC2217066
- DOI: 10.1085/jgp.109.5.633
Separation of gating properties from permeation and block in mslo large conductance Ca-activated K+ channels
Abstract
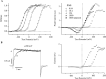
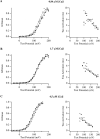
In this and the following paper we have examined the kinetic and steady-state properties of macroscopic mslo Ca-activated K+ currents in order to interpret these currents in terms of the gating behavior of the mslo channel. To do so, however, it was necessary to first find conditions by which we could separate the effects that changes in Ca2+ concentration or membrane voltage have on channel permeation from the effects these stimuli have on channel gating. In this study we investigate three phenomena which are unrelated to gating but are manifest in macroscopic current records: a saturation of single channel current at high voltage, a rapid voltage-dependent Ca2+ block, and a slow voltage-dependent Ba2+ block. Where possible methods are described by which these phenomena can be separated from the effects that changes in Ca2+ concentration and membrane voltage have on channel gating. Where this is not possible, some assessment of the impact these effects have on gating parameters determined from macroscopic current measurements is provided. We have also found that without considering the effects of Ca2+ and voltage on channel permeation and block, macroscopic current measurements suggest that mslo channels do not reach the same maximum open probability at all Ca2+ concentrations. Taking into account permeation and blocking effects, however, we find that this is not the case. The maximum open probability of the mslo channel is the same or very similar over a Ca2+ concentration range spanning three orders of magnitude indicating that over this range the internal Ca2+ concentration does not limit the ability of the channel to be activated by voltage.
Figures







References
-
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
-
- Andersen OS, Procopio J. Ion movement through gramicidin A channels. On the importance of the aqueous diffusion resistance and ion-water interactions. Acta Physiol Scand (Suppl) 1980;481:27–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

