Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels
- PMID: 9154910
- PMCID: PMC2217061
- DOI: 10.1085/jgp.109.5.647
Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels
Abstract
The kinetic and steady-state properties of macroscopic mslo Ca-activated K+ currents were studied in excised patches from Xenopus oocytes. In response to voltage steps, the timecourse of both activation and deactivation, but for a brief delay in activation, could be approximated by a single exponential function over a wide range of voltages and internal Ca2+ concentrations ([Ca]i). Activation rates increased with voltage and with [Ca]i, and approached saturation at high [Ca]i. Deactivation rates generally decreased with [Ca]i and voltage, and approached saturation at high [Ca]i. Plots of the macroscopic conductance as a function of voltage (G-V) and the time constant of activation and deactivation shifted leftward along the voltage axis with increasing [Ca]i. G-V relations could be approximated by a Boltzmann function with an equivalent gating charge which ranged between 1.1 and 1.8 e as [Ca]i varied between 0.84 and 1,000 microM. Hill analysis indicates that at least three Ca2+ binding sites can contribute to channel activation. Three lines of evidence indicate that there is at least one voltage-dependent unimolecular conformational change associated with mslo gating that is separate from Ca2+ binding. (a) The position of the mslo G-V relation does not vary logarithmically with [Ca]i. (b) The macroscopic rate constant of activation approaches saturation at high [Ca]i but remains voltage dependent. (c) With strong depolarizations mslo currents can be nearly maximally activated without binding Ca2+. These results can be understood in terms of a channel which must undergo a central voltage-dependent rate limiting conformational change in order to move from closed to open, with rapid Ca2+ binding to both open and closed states modulating this central step.
Figures








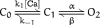
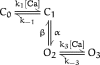









References
-
- Adair GS. The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J Biol Chem. 1925;63:529–545.
-
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
-
- Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the shaker K+channel. Neuron. 1996;16:1169–1177. - PubMed
-
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slolocus. Science (Wash DC) 1991;253:551–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

