BRCA1 is a component of the RNA polymerase II holoenzyme
- PMID: 9159119
- PMCID: PMC20825
- DOI: 10.1073/pnas.94.11.5605
BRCA1 is a component of the RNA polymerase II holoenzyme
Abstract
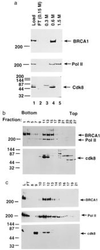
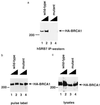
The familial breast-ovarian tumor suppressor gene product BRCA1 was found to be a component of the RNA polymerase II holoenzyme by several criteria. BRCA1 was found to copurify with the holoenzyme over multiple chromatographic steps. Other tested transcription activators that could potentially contact the holoenzyme were not stably associated with the holoenzyme as determined by copurification. Antibody specific for the holoenzyme component hSRB7 specifically purifies BRCA1. Immunopurification of BRCA1 complexes also specifically purifies transcriptionally active RNA polymerase II and transcription factors TFIIF, TFIIE, and TFIIH. Moreover, a BRCA1 domain, which is deleted in about 90% of clinically relevant mutations, participates in binding to the holoenzyme complex in cells. These data are consistent with recent data identifying transcription activation domains in the BRCA1 protein and link the BRCA1 tumor suppressor protein with the transcription process as a holoenzyme-bound protein.
Figures



References
-
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal P A, Harshman K, et al. Science. 1994;266:66–71. - PubMed
-
- Futreal P A, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, et al. Science. 1994;266:120–122. - PubMed
-
- Szabo C I, King M C. Hum Mol Genet. 1995;4:1811–1817. - PubMed
-
- Smith S A, Easton D F, Evans D G R, Ponder B A J. Nat Genet. 1992;2:128–131. - PubMed
-
- Neuhausen S L, Marshall C J. Cancer Res. 1994;54:6069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

