Elevated levels of cysteine protease activity in saliva and salivary glands of the nonobese diabetic (NOD) mouse model for Sjögren syndrome
- PMID: 9159148
- PMCID: PMC20854
- DOI: 10.1073/pnas.94.11.5767
Elevated levels of cysteine protease activity in saliva and salivary glands of the nonobese diabetic (NOD) mouse model for Sjögren syndrome
Abstract
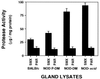
Nonobese diabetic (NOD) mice develop an anti-exocrine gland pathology similar to human Sjögren syndrome. Recently, we demonstrated that NOD-scid mice develop severe loss of submandibular acinar cells with concomitant appearance of abnormal isoforms of salivary proteins suggesting de novo enzymatic cleavage. Because these changes may indicate activation of apoptotic proteases, we examined saliva and salivary tissue for cysteine protease activity. Cysteine protease activities were elevated in saliva and gland lysates from 20-week-old NOD and NOD-scid mice as compared with age- and sex-matched BALB/c or 8-week-old NOD mice. This activity appeared in the submandibular glands, but not in the parotid glands. Western blot analyses using antibodies directed against specific apoptotic proteases (interleukin 1beta converting enzyme, Nedd-2, and Apopain/CPP 32) confirmed these findings. Submandibular glands from NOD-scid mice exhibited the greatest increase in proteolytic activity, indicating that infiltrating leukocytes are not responsible for these changes. Western blot analyses also failed to reveal changes in the levels of cystatins (saliva proteins that inhibit protease activity). Thus, increased cysteine protease activity appears to be directly related to submandibular acinar cell loss in NOD-scid mice involving the apoptotic pathway. Additional protease activity in saliva and gland lysates of older NOD and NOD-scid mice, apparently mutually distinct from cysteine proteases, generated an enzymatically cleaved parotid secretory protein. We suggest, therefore, that proteolytic enzyme activity contributes to loss of exocrine gland tolerance by generating abnormally processed protein constituents.
Figures
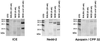

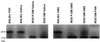
Similar articles
-
Excessive synthesis of matrix metalloproteinases in exocrine tissues of NOD mouse models for Sjögren's syndrome.J Rheumatol. 1998 Dec;25(12):2371-80. J Rheumatol. 1998. PMID: 9858432
-
Aberrant proteolytic digestion of biglycan and decorin by saliva and exocrine gland lysates from the NOD mouse model for autoimmune exocrinopathy.Clin Exp Rheumatol. 2000 Mar-Apr;18(2):233-40. Clin Exp Rheumatol. 2000. PMID: 10812497
-
Salivary gland changes in the NOD mouse model for Sjögren's syndrome: is there a non-immune genetic trigger?Eur J Morphol. 1998 Aug;36 Suppl:247-51. Eur J Morphol. 1998. PMID: 9825931
-
New concepts for the development of autoimmune exocrinopathy derived from studies with the NOD mouse model.Arch Oral Biol. 1999 May;44 Suppl 1:S21-5. doi: 10.1016/s0003-9969(99)00045-x. Arch Oral Biol. 1999. PMID: 10414851 Review.
-
The role of caspase cascade on the development of primary Sjögren's syndrome.J Med Invest. 2003 Feb;50(1-2):32-8. J Med Invest. 2003. PMID: 12630566 Review.
Cited by
-
Associations between metabolic disorders and Sjögren's disease.Jpn Dent Sci Rev. 2024 Dec;60:232-238. doi: 10.1016/j.jdsr.2024.06.002. Epub 2024 Oct 21. Jpn Dent Sci Rev. 2024. PMID: 39502167 Free PMC article. Review.
-
The Critical Biomarkers Identification of Insulin Signaling Involved in Initiating cAMP Signaling Mediated Salivary Secretion in Sjogren Syndrome: Transcriptome Sequencing in NOD Mice Model.Biol Proced Online. 2022 Dec 27;24(1):26. doi: 10.1186/s12575-022-00189-5. Biol Proced Online. 2022. PMID: 36575389 Free PMC article.
-
Nonobese diabetic mice express aspects of both type 1 and type 2 diabetes.Proc Natl Acad Sci U S A. 2006 Aug 15;103(33):12475-80. doi: 10.1073/pnas.0604317103. Epub 2006 Aug 8. Proc Natl Acad Sci U S A. 2006. PMID: 16895987 Free PMC article.
-
Basal lamina disorganisation of the acini and ducts of labial salivary glands from patients with Sjogren's syndrome: association with mononuclear cell infiltration.Ann Rheum Dis. 2006 Feb;65(2):178-83. doi: 10.1136/ard.2004.033837. Epub 2005 Jul 13. Ann Rheum Dis. 2006. PMID: 16014676 Free PMC article.
-
Atrophy of myoepithelial cells in parotid glands of diabetic mice; detection using skeletal muscle actin, a novel marker.FEBS Open Bio. 2013 Feb 1;3:130-4. doi: 10.1016/j.fob.2013.01.009. Print 2013. FEBS Open Bio. 2013. PMID: 23772384 Free PMC article.
References
-
- Gottlieb, P. A., Rossini, A. A. & Mordes, J. P. (1988) Diabetes Care 11, Suppl. 1, 29–36. - PubMed
-
- Hu Y, Nakagawa Y, Purushotham K R, Humphreys-Beher M G. Am J Physiol. 1992;263:E607–E614. - PubMed
-
- Humphreys-Beher M G, Brinkley L, Purushotham K R, Wang P L, Nakagawa Y, Dusek D, Kerr M, Chegini N, Chan E K L. Clin Immunol Immunopathol. 1993;68:350–356. - PubMed
-
- Asamoto H, Oishi M, Okazawa Y, Tochino Y. In: Insulitis and Type 1 Diabetes. Seiichiro T, Tochino Y, Noraka K, editors. Tokyo: Academic; 1986. pp. 61–71.
-
- Fox R I, Kang H-I. Rheum Clin N Am. 1992;18:517–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

