Elevated levels of cysteine protease activity in saliva and salivary glands of the nonobese diabetic (NOD) mouse model for Sjögren syndrome
- PMID: 9159148
- PMCID: PMC20854
- DOI: 10.1073/pnas.94.11.5767
Elevated levels of cysteine protease activity in saliva and salivary glands of the nonobese diabetic (NOD) mouse model for Sjögren syndrome
Abstract
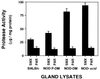
Nonobese diabetic (NOD) mice develop an anti-exocrine gland pathology similar to human Sjögren syndrome. Recently, we demonstrated that NOD-scid mice develop severe loss of submandibular acinar cells with concomitant appearance of abnormal isoforms of salivary proteins suggesting de novo enzymatic cleavage. Because these changes may indicate activation of apoptotic proteases, we examined saliva and salivary tissue for cysteine protease activity. Cysteine protease activities were elevated in saliva and gland lysates from 20-week-old NOD and NOD-scid mice as compared with age- and sex-matched BALB/c or 8-week-old NOD mice. This activity appeared in the submandibular glands, but not in the parotid glands. Western blot analyses using antibodies directed against specific apoptotic proteases (interleukin 1beta converting enzyme, Nedd-2, and Apopain/CPP 32) confirmed these findings. Submandibular glands from NOD-scid mice exhibited the greatest increase in proteolytic activity, indicating that infiltrating leukocytes are not responsible for these changes. Western blot analyses also failed to reveal changes in the levels of cystatins (saliva proteins that inhibit protease activity). Thus, increased cysteine protease activity appears to be directly related to submandibular acinar cell loss in NOD-scid mice involving the apoptotic pathway. Additional protease activity in saliva and gland lysates of older NOD and NOD-scid mice, apparently mutually distinct from cysteine proteases, generated an enzymatically cleaved parotid secretory protein. We suggest, therefore, that proteolytic enzyme activity contributes to loss of exocrine gland tolerance by generating abnormally processed protein constituents.
Figures
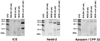

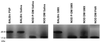
References
-
- Gottlieb, P. A., Rossini, A. A. & Mordes, J. P. (1988) Diabetes Care 11, Suppl. 1, 29–36. - PubMed
-
- Hu Y, Nakagawa Y, Purushotham K R, Humphreys-Beher M G. Am J Physiol. 1992;263:E607–E614. - PubMed
-
- Humphreys-Beher M G, Brinkley L, Purushotham K R, Wang P L, Nakagawa Y, Dusek D, Kerr M, Chegini N, Chan E K L. Clin Immunol Immunopathol. 1993;68:350–356. - PubMed
-
- Asamoto H, Oishi M, Okazawa Y, Tochino Y. In: Insulitis and Type 1 Diabetes. Seiichiro T, Tochino Y, Noraka K, editors. Tokyo: Academic; 1986. pp. 61–71.
-
- Fox R I, Kang H-I. Rheum Clin N Am. 1992;18:517–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

