The neuron-restrictive silencer element: a dual enhancer/silencer crucial for patterned expression of a nicotinic receptor gene in the brain
- PMID: 9159173
- PMCID: PMC20879
- DOI: 10.1073/pnas.94.11.5906
The neuron-restrictive silencer element: a dual enhancer/silencer crucial for patterned expression of a nicotinic receptor gene in the brain
Abstract
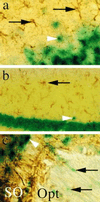
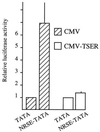
The neuron-restrictive silencer element (NRSE) has been identified in several neuronal genes and confers neuron specificity by silencing transcription in nonneuronal cells. NRSE is present in the promoter of the neuronal nicotinic acetylcholine receptor beta2-subunit gene that determines its neuron-specific expression in the nervous system. Using transgenic mice, we show that NRSE may either silence or enhance transcription depending on the cellular context within the nervous system. In vitro in neuronal cells, NRSE activates transcription of synthetic promoters when located downstream in the 5' untranslated region, or at less than 50 bp upstream from the TATA box, but switches to a silencer when located further upstream. In contrast, in nonneuronal cells NRSE always functions as a silencer. Antisense RNA inhibition shows that the NRSE-binding protein REST contributes to the activation of transcription in neuronal cells.
Figures



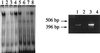
References
-
- Johnson J E, Birren S J, Anderson D J. Nature (London) 1990;346:858–861. - PubMed
-
- Akazawa C, Sasai Y, Nakanishi S, Kageyama R. J Biol Chem. 1992;267:21879–21885. - PubMed
-
- Lee J, Hollenberg S, Snide R L, Turner D, Lipnick N, Weintraub H. Science. 1995;268:836–844. - PubMed
-
- Bessis A, Salmon A, Zoli M, Le Novere N, Picciotto M, Changeux J P. Neuroscience. 1995;69:807–819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

