Neurotrophin regulation of ionic currents and cell size depends on cell context
- PMID: 9159175
- PMCID: PMC20881
- DOI: 10.1073/pnas.94.11.5917
Neurotrophin regulation of ionic currents and cell size depends on cell context
Abstract
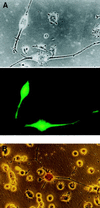
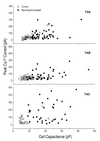
Trk receptor activation by neurotrophins is often considered to have a defined set of actions on target neurons, including supporting neuronal survival, inducing morphological differentiation, and regulating a host of target genes that specify neuronal phenotype. It is not known if all such regulatory effects are obligatory, or if some may vary depending on the cell context in which the receptors are expressed. We have examined this issue by comparing neurotrophin effects on the regulation of electrical excitability and morphological differentiation in two strains of PC12 cells. We found that while neurotrophins induced neurite extension and increased calcium currents in both PC12 cell types, sodium current levels were regulated in only one of these strains. Moreover, we found little correlation between calcium current levels and the extent of morphological differentiation when compared in individual cells of a single strain. Thus, the regulatory effects of neurotrophins on cell phenotype are not fully determined by the Trk receptors that they activate; rather, they can vary with differences in cell context that arise not only between different cell lineages, but also between individual cells of clonal relation.
Figures

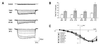

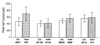
References
-
- Temple S, Qian X. Neuron. 1995;15:249–252. - PubMed
-
- McConnell S K. Neuron. 1995;15:761–768. - PubMed
-
- Ghosh A, Greenberg M E. Neuron. 1995;15:89–103. - PubMed
-
- Vicario-Abejon C, Johe K K, Hazel T G, Collazo D, McKay R D G. Neuron. 1995;15:105–114. - PubMed
-
- Davis A A, Temple S. Nature (London) 1994;372:263–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

