Intrinsic signals in the unique domain target p56(lck) to the plasma membrane independently of CD4
- PMID: 9166404
- PMCID: PMC2136224
- DOI: 10.1083/jcb.137.5.1029
Intrinsic signals in the unique domain target p56(lck) to the plasma membrane independently of CD4
Abstract
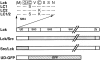
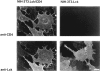
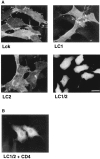
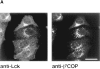
In T lymphocytes, the Src-family protein tyrosine kinase p56(lck) (Lck) is mostly associated with the cytoplasmic face of the plasma membrane. To determine how this distribution is achieved, we analyzed the location of Lck in lymphoid and in transfected nonlymphoid cells by immunofluorescence. We found that in T cells Lck was targeted correctly, independently of the cell surface proteins CD4 and CD8 with which it interacts. Similarly, in transfected NIH-3T3 fibroblasts, Lck was localized at the plasma membrane, indicating that T cell-specific proteins are not required for targeting. Some variation in subcellular distribution was observed when Lck was expressed in HeLa and MDCK cells. In these cells, Lck associated with both the plasma membrane and the Golgi apparatus, while subsequent expression of CD4 resulted in the loss of Golgi-associated staining. Together, these data indicate that Lck contains intrinsic signals for targeting to the plasma membrane. Furthermore, delivery to this site may be achieved via association with exocytic transport vesicles. A mutant Lck molecule in which the palmitoylation site at cysteine 5 was changed to lysine (LC2) localized to the plasma membrane and the Golgi region in NIH3T3 cells. However, the localization of a mutant in which the palmitoylation site at cysteine 3 was changed to serine (LC1) was indistinguishable from wild-type Lck. Chimeras composed of only the unique domain of Lck linked to either c-Src or the green fluorescent protein similarly localized to the plasma membrane of NIH-3T3 cells. Thus, the targeting of Lck appears to be determined primarily by its unique domain and may be influenced by the use of different palmitoylation sites.
Figures






References
-
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem. 1994;24:16701–16705. - PubMed
-
- August A, Dupont B. Activation of Src family kinase Lck following CD28 crosslinking in the Jurkat leukemic cell line. Biochem Biophys Res Commun. 1994;199:1466–1473. - PubMed
-
- Bar SD, Rotin D, Batzer A, Mandiyan V, Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993;74:83–91. - PubMed
-
- Berthiaume L, Resh MD. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

