A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation
- PMID: 9166406
- PMCID: PMC2136220
- DOI: 10.1083/jcb.137.5.1057
A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation
Abstract
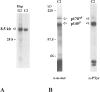
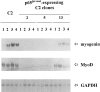
As a rule, hepatocyte growth factor/scatter factor (HGF/SF) is produced by mesenchymal cells, while its receptor, the tyrosine kinase encoded by the met proto-oncogene, is expressed by the neighboring epithelial cells in a canonical paracrine fashion. In the present work we show that both HGF/SF and met are coexpressed by undifferentiated C2 mouse myoblasts. In growing cells, the autocrine loop is active as the receptor exhibits a constitutive phosphorylation on tyrosine that can be abrogated by exogenously added anti-HGF/SF neutralizing antibodies. The transcription of HGF/SF and met genes is downregulated when myoblasts stop proliferating and differentiate. The coexpression of HGF/SF and met genes is not exclusive to C2 cells since it has been assessed also in other myogenic cell lines and in mouse primary satellite cells, suggesting that HGF/SF could play a role in muscle development through an autocrine way. To analyze the biological effects of HGF/SF receptor activation, we stably expressed the constitutively activated receptor catalytic domain (p65(tpr-met)) in C2 cells. This active kinase determined profound changes in cell shape and inhibited myogenesis at both morphological and biochemical levels. Notably, a complete absence of muscle regulatory markers such as MyoD and myogenin was observed in p65(tpr-met) highly expressing C2 clones. We also studied the effects of the ectopic expression of human isoforms of met receptor (h-met) and of HGF/SF (h-HGF/SF) in stable transfected C2 cells. Single constitutive expression of h-met or h-HGF/SF does not alter substantially the growth and differentiation properties of the myoblast cells, probably because of a species-specific ligand-receptor interaction. A C2 clone expressing simultaneously both h-met and h-HGF/SF is able to grow in soft agar and shows a decrease in myogenic potential comparable to that promoted by p65(tpr-met) kinase. These data indicate that a met kinase signal released from differentiation-dependent control provides a negative stimulus for the onset of myogenic differentiation.
Figures






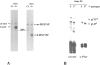
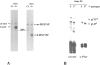
References
-
- Adams JC, Furlong RA, Watt FM. Production of scatter factor by ndk, a strain of epithelial cells, and inhibition of scatter factor activity by suramin. J Cell Sci. 1991;98:385–394. - PubMed
-
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. - PubMed
-
- Bellusci S, Moens G, Gaudino G, Comoglio PM, Nakamura T, Thiery J-P, Jouanneau J. Creation of an hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene. 1994;9:1091–1099. - PubMed
-
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-metreceptor in the migration of myogenic precursor cells into the limb bud. Nature (Lond) 1995;376:768–771. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

