Human dendritic cells skew isotype switching of CD40-activated naive B cells towards IgA1 and IgA2
- PMID: 9166420
- PMCID: PMC2196343
- DOI: 10.1084/jem.185.11.1909
Human dendritic cells skew isotype switching of CD40-activated naive B cells towards IgA1 and IgA2
Abstract
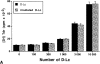
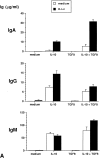
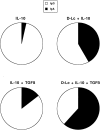
Within T cell-rich areas of secondary lymphoid organs, interdigitating dendritic cells recruit antigen-specific T cells that then induce B cells to secrete Igs. This study investigates the possible role(s) of dendritic cells in the regulation of human B cell responses. In the absence of exogenous cytokines, in vitro generated dendritic cells (referred to as Dendritic Langerhans cells, D-Lc) induced surface IgA expression on approximately 10% of CD40-activated naive sIgD+ B cells. In the presence of IL-10 and TGF-beta, a combination of cytokines previously identified for its capacity to induce IgA switch, D-Lc strongly potentiated the induction of sIgA on CD40-activated naive B cells from 5% to 40-50%. D-Lc alone did not induce the secretion of IgA by CD40-activated naive B cells, which required further addition of IL-10. Furthermore, D-Lc skewed towards the IgA isotype at the expense of IgG, the Ig production of CD40-activated naive B cells cultured in the presence of IL-10 and TGF-beta. Importantly, under these culture conditions, both IgA1 and IgA2 were detected. In the presence of IL-10, secretion of IgA2 by CD40-activated naive B cells could be detected only in response to D-Lc and was further enhanced by TGF-beta. Collectively, these results suggest that in addition to activating T cells in the extrafollicular areas of secondary lymphoid organs, human D-Lc also directly modulate T cell-dependent B cell growth and differentiation, by inducing the IgA isotype switch.
Figures













References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

