Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors
- PMID: 9169514
- PMCID: PMC6573334
- DOI: 10.1523/JNEUROSCI.17-12-04536.1997
Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors
Abstract
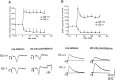
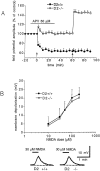
Dopamine D2 receptors (D2Rs) are of crucial importance in the striatal processing of motor information received from the cortex. Disruption of the D2R gene function in mice results in a severe locomotor impairment. This phenotype has analogies with Parkinson's disease symptoms. D2R-null mice were used to investigate the role of this receptor in the generation of striatal synaptic plasticity. Tetanic stimulation of corticostriatal fibers produced long-term depression (LTD) of EPSPs in slices from wild-type (WT) mice. Strikingly, recordings from D2R-null mice showed the converse: long-term potentiation (LTP). This LTP, unlike LTD, was blocked by an NMDA receptor antagonist. In magnesium-free medium, LTP was also revealed in WT mice and found to be enhanced by L-sulpiride, a D2R antagonist, whereas it was reversed into LTD by LY 17555, a D2R agonist. In D2R-null mice this modulation was lost. Thus, our study indicates that D2Rs play a key role in mechanisms underlying the direction of long-term changes in synaptic efficacy in the striatum. It also shows that an imbalance between D2R and NMDA receptor activity induces altered synaptic plasticity at corticostriatal synapses. This abnormal synaptic plasticity might cause the movement disorders observed in Parkinson's disease.
Figures



References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, prefrontal and limbic functions. Prog Brain Res. 1990;85:119–146. - PubMed
-
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. - PubMed
-
- Artola A, Hensch T, Singer W. Calcium-induced long-term depression in the visual cortex of the rat in vitro. J Neurophysiol. 1996;76:984–994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
