Heterozygous peripheral myelin protein 22-deficient mice are affected by a progressive demyelinating tomaculous neuropathy
- PMID: 9169527
- PMCID: PMC6573352
- DOI: 10.1523/JNEUROSCI.17-12-04662.1997
Heterozygous peripheral myelin protein 22-deficient mice are affected by a progressive demyelinating tomaculous neuropathy
Abstract
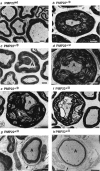
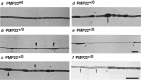
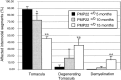
Hereditary neuropathy with liability to pressure palsy (HNPP) is associated with a heterozygous 1.5 megabase deletion on chromosome 17 that includes the peripheral myelin protein (PMP) gene PMP22. We show that heterozygous PMP22 knock-out mice, which carry only one functional pmp22 allele and thus genetically mimic HNPP closely, display similar morphological and electrophysiological features as observed in HNPP nerves. As reported previously, focal hypermyelinating structures called tomacula, the pathological hallmarks of HNPP, develop progressively in young PMP22(+/0) mice. By following the fate of tomacula during aging, we demonstrate now that these mutant animals are also interesting models for examining HNPP disease mechanisms. Subtle electrophysiological abnormalities are detected in PMP22(+/0) mice >1 year old, and a significant number of abnormally swollen and degenerating tomacula are present. Thinly myelinated axons and supernumerary Schwann cells forming onion bulbs as fingerprints of repeated cycles of demyelination and remyelination are also encountered frequently. Quantitative analyses using electron microscopy on cross sections and light microscopy on single teased nerve fibers suggest that tomacula are intrinsically unstable structures that are prone to degeneration; however, the severity of morphological and electrophysiological abnormalities in PMP22(+/0) mice is variable. These combined findings are reminiscent of the disease progression in HNPP and offer a possible explanation about why some HNPP patients develop a chronic motor and sensory neuropathy later in life that resembles demyelinating forms of Charcot-Marie-Tooth disease by both morphological and clinical criteria.
Figures





References
-
- Adlkofer K, Martini R, Aguzzi A, Zielasek J, Toyka KV, Suter U. Hypermyelination and demyelinating peripheral neuropathy in pmp22-deficient mice. Nature Genet. 1995;11:274–280. - PubMed
-
- Amato AA, Gronseth GS, Callerame KJ, Kagan-Hallet KS, Bryan WW, Barohn RJ. Tomaculous neuropathy: a clinical and electrophysiological study in patients with and without 1.5-Mb deletions in chromosome 17p11.2. Muscle Nerve. 1996;19:16–22. - PubMed
-
- Chance PF, Alderson MK, Leppig KA, Lensch MW, Matsunami N, Smith B, Swanson PD, Odelberg SJ, Disteche CM, Bird TD. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 1993;72:143–151. - PubMed
-
- Davies DM. Recurrent peripheral nerve palsies in a family. Lancet. 1954;2:266–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
