The shaking-B2 mutation disrupts electrical synapses in a flight circuit in adult Drosophila
- PMID: 9169530
- PMCID: PMC6573327
- DOI: 10.1523/JNEUROSCI.17-12-04700.1997
The shaking-B2 mutation disrupts electrical synapses in a flight circuit in adult Drosophila
Abstract
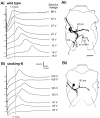
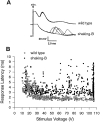
The shaking-B2 mutation was used to analyze synapses between haltere afferents and a flight motoneuron in adult Drosophila. We show that the electrical synapses among many neurons in the flight circuit are disrupted in shaking-B2 flies, suggesting that shaking-B expression is required for electrical synapses throughout the nervous system. In wild-type flies haltere afferents are dye-coupled to the first basalar motoneuron, and stimulation of these afferents evokes electromyograms from the first basalar muscle with short latencies. In shaking-B2 flies dye coupling between haltere afferents and the motoneuron is abolished, and afferent stimulation evokes electromyograms at abnormally long latencies. Intracellular recordings from the motoneuron confirm that the site of the defect in shaking-B2 flies is at the synapses between haltere afferents and the flight motoneuron. The nicotinic cholinergic antagonist mecamylamine blocks the haltere-to-flight motoneuron synapses in shaking-B2 flies but does not block those synapses in wild-type flies. Together, these results show that the haltere-to-flight motoneuron synapses comprise an electrical component that requires shaking-B and a chemical component that is likely to be cholinergic.
Figures



References
-
- Baird DH, Koto M, Wyman RJ. Dendritic reduction in Passover, a Drosophila mutant with defective giant fiber neuronal pathway. J Neurobiol. 1993;24:971–984. - PubMed
-
- Balakrishnan R, Rodrigues V. The shaker and shaking-B genes specify elements in the processing of gustatory information in Drosophila melanogaster. J Exp Biol. 1991;157:161–181. - PubMed
-
- Bennett MVL. Electrical transmission: a functional analysis and comparison to chemical transmission. In: Brookhart JM, Mountcastle VB, editors. Handbook of physiology: the nervous system. American Physiological Society; Bethesda, MD: 1977. pp. 357–416.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
