Treatment of patent ductus arteriosus with ibuprofen
- PMID: 9175948
- PMCID: PMC1720646
- DOI: 10.1136/fn.76.3.f179
Treatment of patent ductus arteriosus with ibuprofen
Abstract
Aim: To evaluate the efficiency and side effects of ibuprofen for the early treatment of patent ductus arteriosus (PDA) and compare it with indomethacin.
Methods: Forty preterm infants with gestational ages of less than 33 weeks, with respiratory distress syndrome (RDS) and echocardiographically confirmed PDA, were randomly assigned at days 2 to 3 of life to receive either intravenous indomethacin 3 x 0.2 mg/kg at 12 hour intervals or intravenous ibuprofen 1 x 10 mg/kg, followed by 5 mg/kg 24 and 48 hours later.
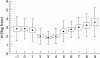
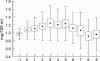
Results: PDA closed in 15 of 20 patients from the indomethacin group (75%) and in 16 of 20 (80%) from the ibuprofen group. Seven patients (three indomethacin, four ibuprofen) required a second treatment with indomethacin and in five (three in the indomethacin group and two in the ibuprofen group) the duct was ultimately ligated. Ibuprofen patients had a better urinary output and showed no increase in serum creatinine concentrations compared with the indomethacin group. Ibuprofen was not associated with any other side effect.
Conclusions: Ibuprofen treatment seems to be as efficient as indomethacin in closing PDA on the third day of life in preterm infants with respiratory distress syndrome and seems to have fewer renal side effects.
Figures


Comment in
-
Is ibuprofen a useful treatment for patent ductus arteriosus?Arch Dis Child Fetal Neonatal Ed. 1998 Jan;78(1):F80. doi: 10.1136/fn.78.1.f78e. Arch Dis Child Fetal Neonatal Ed. 1998. PMID: 9536853 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
