Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner
- PMID: 9177166
- PMCID: PMC20998
- DOI: 10.1073/pnas.94.12.6048
Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner
Abstract
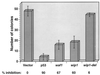
Exposure of mammalian cells to ionizing radiation (IR) induces a complex array of cellular responses including cell cycle arrest and/or apoptosis. IR-induced G1 arrest has been shown to depend on the presence of the tumor suppressor p53, which acts as a transcriptional activator of several genes. p53 also plays a role in the induction of apoptosis in response to DNA damage, and this pathway can be activated by both transcription-dependent and -independent mechanisms. Here we report the identification of a novel transcript whose expression is induced in response to IR in a p53-dependent manner, and that shows homology to the type 2C protein phosphatases. We have named this novel gene, wip1. In vitro, recombinant Wip1 displayed characteristics of a type 2C phosphatase, including Mg2+ dependence and relative insensitivity to okadaic acid. Studies performed in several cell lines revealed that wip1 accumulation following IR correlates with the presence of wild-type p53. The accumulation of wip1 mRNA following IR was rapid and transient, and the protein was localized to the nucleus. Similar to waf1, ectopic expression of wip1 in human cells suppressed colony formation. These results suggest that Wip1 might contribute to growth inhibitory pathways activated in response to DNA damage in a p53-dependent manner.
Figures



References
-
- Hollestein M, Sidransky D, Vogelstein B, Harris C C. Science. 1991;253:49–53. - PubMed
-
- Bates S, Vousden K H. Curr Opin Genet Dev. 1996;6:12–19. - PubMed
-
- Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Nature (London) 1992;356:215–221. - PubMed
-
- Kastan M B, Zhan Q, El-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J. Cell. 1992;71:587–597. - PubMed
-
- Lowe S W, Schmitt S W, Smith B A, Osborne B A, Jacks T. Nature (London) 1993;362:847–849. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

