Iron-sulfur cluster disassembly in the FNR protein of Escherichia coli by O2: [4Fe-4S] to [2Fe-2S] conversion with loss of biological activity
- PMID: 9177174
- PMCID: PMC21006
- DOI: 10.1073/pnas.94.12.6087
Iron-sulfur cluster disassembly in the FNR protein of Escherichia coli by O2: [4Fe-4S] to [2Fe-2S] conversion with loss of biological activity
Abstract
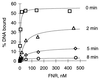
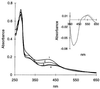
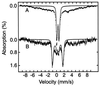
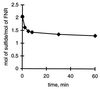
The transcription factor FNR (fumarate nitrate reduction) requires the presence of an iron-sulfur (Fe-S) cluster for its function as a global transcription regulator in Escherichia coli when oxygen becomes scarce. To define the oxidation state and type of Fe-S cluster present in the active form of FNR, we have studied anaerobically purified FNR with Mössbauer spectroscopy. Our data showed that this form of FNR contained a [4Fe-4S]2+ cluster (delta = 0.45 mm/s; DeltaEQ = 1.22 mm/s) and that the [4Fe-4S]2+ cluster was rapidly destroyed on exposure of FNR to air. Under these conditions, the yellow-green active form of FNR turned deep red; analysis of sulfide indicated that 70% of the labile sulfide was still present, suggesting that the Fe-S cluster had been converted into a different form. Little [3Fe-4S] cluster was, however, detected by EPR. According to Mössbauer spectroscopy, the [4Fe-4S]2+ cluster was converted in about 60% yield to a [2Fe-2S]2+ cluster (delta = 0.28 mm/s; DeltaEQ = 0.58 mm/s) following 17 min of exposure to air. The [2Fe-2S]2+ cluster form of FNR was much more stable to oxygen, but was unable to sustain biological activity (e.g., DNA binding). However, DNA binding and the absorption spectrum characteristic of the [4Fe-4S]2+ cluster could be largely restored from the [2Fe-2S]2+ form when Cys, Fe, DTT, and the NifS protein were added. It has yet to be determined whether the form of FNR containing the [2Fe-2S]2+ cluster has any biological significance, e.g., as an in vivo intermediate that is more rapidly converted to the active form than the apoprotein.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

