Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells
- PMID: 9177196
- PMCID: PMC21028
- DOI: 10.1073/pnas.94.12.6210
Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells
Abstract
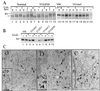
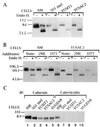
The loss of tyrosinase, the key enzyme in melanin synthesis, has been implicated in the dedifferentiation of malignant melanocytes. The presence of tyrosinase transcripts and antigenic peptides in melanoma tumors prompted us to investigate whether the basis for the loss of the enzyme was proteolytic degradation. Toward this aim, we followed the kinetics of synthesis, degradation, processing, chaperone binding, inhibitor sensitivity, and subcellular localization of tyrosinase in normal and malignant melanocytes. We found that, in amelanotic melanoma cell lines, tyrosinase failed to reach the melanosome, the organelle for melanin synthesis, because it was retained in the endoplasmic reticulum (ER) and then degraded. Tyrosinase appeared mostly as a 70-kDa core-glycosylated, endoglycosidase H-sensitive, immature form bound to the ER chaperone calnexin and had a life-span of only 25% of normal. Maturation and transit from the ER to the Golgi compartment was facilitated by lowering the temperature of incubation to 31 degrees C. Several proteasome inhibitors caused the accumulation of an approximately 60-kDa tyrosinase doublet that was more prominent in malignant than in normal melanocytes and promoted, to various degrees, the maturation of tyrosinase in melanoma cells and the translocation of the enzyme to melanosomes. The appearance of ubiquitinated tyrosinase after treatment of normal melanocytes with N-acetyl-L-leucinyl-L-leucinal-L-norleucinal reinforced our notion that some tyrosinase is normally degraded by proteasomes. Proteolysis of tyrosinase by proteasomes is consistent with the production of antigenic tyrosinase peptides that are presented to the immune system by major histocompatibility complex class I molecules.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

