The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ
- PMID: 9177213
- PMCID: PMC21045
- DOI: 10.1073/pnas.94.12.6307
The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ
Abstract
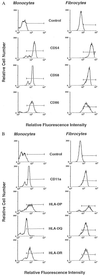
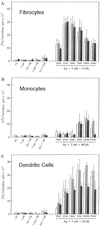
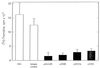
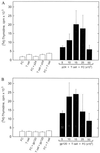
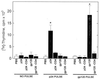
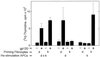
Recent studies have identified a novel population of blood-borne cells, termed fibrocytes, that have a distinct cell surface phenotype (collagen+/CD13(+)/CD34(+)/CD45(+)), rapidly enter sites of tissue injury, and synthesize connective tissue matrix molecules. We found by flow cytometry that purified human fibrocytes express each of the known surface components that are required for antigen presentation, including class II major histocompatability complex molecules (HLA-DP, -DQ, and -DR), the costimulatory molecules CD80 and CD86, and the adhesion molecules CD11a, CD54, and CD58. Human fibrocytes induced antigen-presenting cell-dependent T cell proliferation when cultured with specific antigen and this proliferative activity was significantly higher than that induced by monocytes and nearly as high as that induced by purified dendritic cells. Mouse fibrocytes also were found to express the surface components required for antigen presentation and to function as potent APCs in vitro. Mouse fibrocytes pulsed in vitro with the HIV-proteins p24 or gp120 and delivered to a site of cutaneous injury were found to migrate to proximal lymph nodes and to specifically prime naive T cells. These data suggest that fibrocytes play an early and important role in the initiation of antigen-specific immunity.
Figures

References
-
- Mast B A. In: Wound Healing: Biochemical and Clinical Aspects. Cohen I K, Diegelmann R F, Lindblad W J, editors. Philadelphia: Saunders; 1992. pp. 344–355.
-
- Davidson J M. In: Inflammation: Basic Principles and Clinical Correlates. Gallin J I, Goldstein I M, Snyderman R, editors. New York: Raven; 1992. pp. 809–819.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

