Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis
- PMID: 9177214
- PMCID: PMC21046
- DOI: 10.1073/pnas.94.12.6313
Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis
Abstract
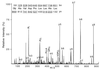
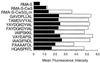
HLA class I molecules bind peptides derived from proteins degraded in the cytoplasm and display them for surveillance by the immune system. The recognition of HLA class I molecules by natural killer (NK) cells generally inhibits the lytic process. To investigate the role of peptides in the interaction between HLA class I molecules and NK receptors, we first had to identify representative endogenous peptides. Individual peptides bound to HLA-Cw*0304 were isolated and sequenced by tandem mass spectrometry. These peptides ranged in length from 8 to 11 residues and shared an alanine at position 2 and a C-terminal leucine. The murine transporters associated with antigen processing (TAP)-deficient cell line RMA-S was transfected with HLA-Cw*0304 to test whether HLA molecules loaded with a single peptide could deliver the inhibitory signal to NK cells expressing p58.2, which is a killer cell inhibitory receptor known to interact with HLA molecules bearing the HLA-Cw3 public epitope. We found that, in the absence of exogenous peptides, the HLA-Cw*0304 transfectants were killed at levels comparable to untransfected RMA-S cells whereas protection from lysis required both HLA-Cw*0304 heavy chain expression and an exogenously added HLA-Cw*0304-binding peptide. Importantly, not only were HLA-Cw*0304-binding peptides required for protection, but the ability of individual peptides to provide protection differed widely. These studies indicate that the ability to distinguish between subsets of peptides may be a general feature of HLA class I recognition by NK cells.
Figures


References
-
- Bjorkman P J, Parham P. Annu Rev Biochem. 1990;59:253–288. - PubMed
-
- Madden D R. Annu Rev Immunol. 1995;13:587–622. - PubMed
-
- York I A, Rock K L. Annu Rev Immunol. 1996;14:369–396. - PubMed
-
- Gumperz J E, Parham P. Nature (London) 1995;378:245–248. - PubMed
-
- Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari M C, Moretta L. Annu Rev Immunol. 1996;14:619–648. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

