The epitopes for natural polyreactive antibodies are rich in proline
- PMID: 9177218
- PMCID: PMC21050
- DOI: 10.1073/pnas.94.12.6335
The epitopes for natural polyreactive antibodies are rich in proline
Abstract
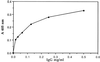
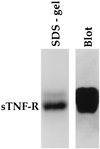
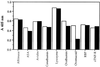
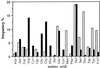
"Natural" polyreactive antibodies, which bind in a nonspecific manner to a range of biological molecules both of self- and nonself- origin, are normal constituents of serum and are a significant part of the immune repertoire in many species, including humans. Autoantibodies to sTNF-R (the 55-kDa extracellular domain of the human receptor to tumor necrosis factor alpha) were affinity purified from normal human sera using immobilized sTNF-R. The isolated anti-sTNF-R IgG bound both native and denatured forms of the receptor with low affinity. These antibodies also bound to different proteins and therefore are considered to be polyreactive. We used the anti-sTNF-R antibodies and purified polyreactive antibodies to mannose-specific lectin from garlic (Allium sativum) for screening a peptide library displayed on filamentous M13 phage. After the biopanning procedure, we failed to find epitopes with a consensus sequence; however, we found that proline is the most frequent amino acid in the selected phagotopes. Proline is commonly present at solvent-exposed sites in proteins, such as loops, turns, N-terminal first turn of helix, and random coils. Thus, structures containing proline can serve as conformation-dependent common "public" epitopes for polyreactive natural antibodies. Our findings may be important for understanding polyreactivity in general and for the significance of polyreactive natural antibodies in immunological homeostasis.
Figures


Similar articles
-
Natural antibodies to dietary proteins: the existence of natural antibodies to alliinase (Alliin lyase) and mannose-specific lectin from garlic (Allium sativum) in human serum.Immunol Lett. 1995 Jul-Aug;47(1-2):53-7. doi: 10.1016/0165-2478(95)00067-f. Immunol Lett. 1995. PMID: 8537101
-
Natural human antibodies to dietary lectins.FEBS Lett. 1996 Nov 18;397(2-3):139-42. doi: 10.1016/s0014-5793(96)01154-4. FEBS Lett. 1996. PMID: 8955334
-
Natural antibodies against alliinase in human serum and polyclonal antibodies elicited in rabbit share the same immunogenic determinants.Immunol Lett. 2000 Jan 10;71(1):43-7. doi: 10.1016/s0165-2478(99)00162-5. Immunol Lett. 2000. PMID: 10709784
-
The humoral immune response in autoimmunity.Dermatol Clin. 1993 Jul;11(3):379-89. Dermatol Clin. 1993. PMID: 8365026 Review.
-
Antibody polyreactivity in health and disease: statu variabilis.J Immunol. 2013 Aug 1;191(3):993-9. doi: 10.4049/jimmunol.1300880. J Immunol. 2013. PMID: 23873158 Review.
Cited by
-
Phage-displayed T-cell epitope grafted into immunoglobulin heavy-chain complementarity-determining regions: an effective vaccine design tested in murine cysticercosis.Infect Immun. 1999 Sep;67(9):4764-70. doi: 10.1128/IAI.67.9.4764-4770.1999. Infect Immun. 1999. PMID: 10456929 Free PMC article.
-
Identification of amino acid residue in the Cronobacter sakazakii LamB responsible for the receptor compatibility of polyvalent coliphage CSP1.J Virol. 2024 Oct 22;98(10):e0067624. doi: 10.1128/jvi.00676-24. Epub 2024 Sep 9. J Virol. 2024. PMID: 39248490 Free PMC article.
-
A proline-rich region with a highly periodic sequence in Streptococcal beta protein adopts the polyproline II structure and is exposed on the bacterial surface.J Bacteriol. 2002 Nov;184(22):6376-83. doi: 10.1128/JB.184.22.6376-6393.2002. J Bacteriol. 2002. PMID: 12399508 Free PMC article.
-
Diagnostic Profiling of the Human Public IgM Repertoire With Scalable Mimotope Libraries.Front Immunol. 2019 Dec 3;10:2796. doi: 10.3389/fimmu.2019.02796. eCollection 2019. Front Immunol. 2019. PMID: 31849974 Free PMC article.
-
One day is enough: rapid and specific host-parasite interactions in a stickleback-trematode system.Biol Lett. 2006 Sep 22;2(3):382-4. doi: 10.1098/rsbl.2006.0462. Biol Lett. 2006. PMID: 17148409 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

