CCAAT/enhancer binding protein epsilon is preferentially up-regulated during granulocytic differentiation and its functional versatility is determined by alternative use of promoters and differential splicing
- PMID: 9177240
- PMCID: PMC21072
- DOI: 10.1073/pnas.94.12.6462
CCAAT/enhancer binding protein epsilon is preferentially up-regulated during granulocytic differentiation and its functional versatility is determined by alternative use of promoters and differential splicing
Abstract
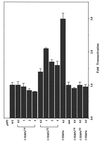
CCAAT/enhancer binding protein (C/EBP) epsilon is a recently cloned member of the C/EBP family of transcription factors and is expressed exclusively in cells of hematopoietic origin. The human C/EBPepsilon gene is transcribed by two alternative promoters, Palpha and Pbeta. A combination of differential splicing and alternative use of promoters generates four mRNA isoforms, of 2.6 kb and 1.3-1.5 kb in size. These transcripts can encode three proteins of calculated molecular mass 32.2 kDa, 27.8 kDa, and 14.3 kDa. Accordingly, Western blots with antibodies specific for the DNA-binding domain, that is common to all forms, identify multiple proteins. C/EBPepsilon mRNA was greatly induced during in vitro granulocytic differentiation of human primary CD34(+) cells. Retinoic acid treatment of HL60 promyelocytic leukemia cells for 24 hr induced C/EBPepsilon mRNA levels by 4-fold, while prolonged treatment gradually reduced mRNA expression to pretreatment levels. Transient transfection experiments with expression vectors for two of the isoforms demonstrated that the 32.2-kDa protein is an activator of transcription of granulocyte colony-stimulating factor receptor promoter, while the 14.3-kDa protein is not. Thus, C/EBPepsilon is regulated in a complex fashion and may play a role in the regulation of genes involved in myeloid differentiation.
Figures




References
-
- Ness S A, Engel J D. Curr Opin Genet Dev. 1994;4:718–724. - PubMed
-
- Shivdasani R A, Orkin S H. Blood. 1996;87:4025–4039. - PubMed
-
- Tenen, D. G., Hromas, R., Licht, J. & Zhang, D.-E. (1997) Blood, in press. - PubMed
-
- Scott L M, Civin C I, Rorth P, Friedman A D. Blood. 1992;80:1725–1735. - PubMed
-
- Wedel A, Ziegler-Heitbrock H W L. Immunobiology. 1995;193:171–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

