Integral membrane proteins of the nuclear envelope are dispersed throughout the endoplasmic reticulum during mitosis
- PMID: 9182656
- PMCID: PMC2132536
- DOI: 10.1083/jcb.137.6.1199
Integral membrane proteins of the nuclear envelope are dispersed throughout the endoplasmic reticulum during mitosis
Abstract
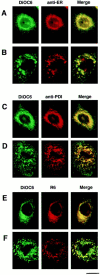
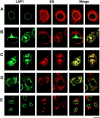
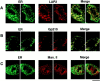
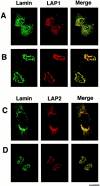
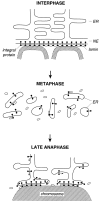
We have analyzed the fate of several integral membrane proteins of the nuclear envelope during mitosis in cultured mammalian cells to determine whether nuclear membrane proteins are present in a vesicle population distinct from bulk ER membranes after mitotic nuclear envelope disassembly or are dispersed throughout the ER. Using immunofluorescence staining and confocal microscopy, we compared the localization of two inner nuclear membrane proteins (laminaassociated polypeptides 1 and 2 [LAP1 and LAP2]) and a nuclear pore membrane protein (gp210) to the distribution of bulk ER membranes, which was determined with lipid dyes (DiOC6 and R6) and polyclonal antibodies. We found that at the resolution of this technique, the three nuclear envelope markers become completely dispersed throughout ER membranes during mitosis. In agreement with these results, we detected LAP1 in most membranes containing ER markers by immunogold electron microscopy of metaphase cells. Together, these findings indicate that nuclear membranes lose their identity as a subcompartment of the ER during mitosis. We found that nuclear lamins begin to reassemble around chromosomes at the end of mitosis at the same time as LAP1 and LAP2 and propose that reassembly of the nuclear envelope at the end of mitosis involves sorting of integral membrane proteins to chromosome surfaces by binding interactions with lamins and chromatin.
Figures
References
-
- Burke B. On the cell-free association of lamins A and C with metaphase chromosomes. Exp Cell Res. 1990;186:169–176. - PubMed
-
- Burke B, Gerace L. A cell-free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986;44:639–652. - PubMed
-
- Foisner R, Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell. 1993;73:1267–1279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

