Possible involvement of phosphorylation of occludin in tight junction formation
- PMID: 9182670
- PMCID: PMC2132539
- DOI: 10.1083/jcb.137.6.1393
Possible involvement of phosphorylation of occludin in tight junction formation
Abstract
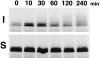
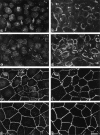
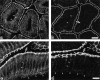
Occludin is an integral membrane protein localizing at tight junctions in epithelial and endothelial cells. Occludin from confluent culture MDCK I cells resolved as several (>10) bands between 62 and 82 kD in SDS-PAGE, of which two or three bands of the lowest Mr were predominant. Among these bands, the lower predominant bands were essentially extracted with 1% NP-40, whereas the other higher Mr bands were selectively recovered in the NP-40-insoluble fraction. Alkaline phosphatase treatment converged these bands of occludin both in NP-40-soluble and -insoluble fractions into the lowest Mr band, and phosphoamino acid analyses identified phosphoserine (and phosphothreonine weakly) in the higher Mr bands of occludin. These findings indicated that phosphorylation causes an upward shift of occludin bands and that highly phosphorylated occludin resists NP-40 extraction. When cells were grown in low Ca medium, almost all occludin was NP-40 soluble. Switching from low to normal Ca medium increased the amount of NP-40-insoluble occludin within 10 min, followed by gradual upward shift of bands. This insolubilization and the band shift correlated temporally with tight junction formation detected by immunofluorescence microscopy. Furthermore, we found that the anti-chicken occludin mAb, Oc-3, did not recognize the predominant lower Mr bands of occludin (non- or less phosphorylated form) but was specific to the higher Mr bands (phosphorylated form) on immunoblotting. Immunofluorescence microscopy revealed that this mAb mainly stained the tight junction proper of intestinal epithelial cells, whereas other anti-occludin mAbs, which can recognize the predominant lower Mr bands, labeled their basolateral membranes (and the cytoplasm) as well as tight junctions. Therefore, we conclude that non- or less phosphorylated occludin is distributed on the basolateral membranes and that highly phosphorylated occludin is selectively concentrated at tight juctions as the NP-40-insoluble form. These findings suggest that the phosphorylation of occludin is a key step in tight junction assembly.
Figures





Similar articles
-
Phosphorylation of occludin correlates with occludin localization and function at the tight junction.Am J Physiol. 1997 Dec;273(6):C1859-67. doi: 10.1152/ajpcell.1997.273.6.C1859. Am J Physiol. 1997. PMID: 9435490
-
Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins.Am J Physiol. 1999 May;276(5):F737-50. doi: 10.1152/ajprenal.1999.276.5.F737. Am J Physiol. 1999. PMID: 10330056
-
Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells.J Membr Biol. 1999 Jul 15;170(2):147-56. doi: 10.1007/s002329900544. J Membr Biol. 1999. PMID: 10430658
-
Molecular dissection of tight junctions.Cell Struct Funct. 1996 Oct;21(5):381-5. doi: 10.1247/csf.21.381. Cell Struct Funct. 1996. PMID: 9118244 Review.
-
Breaking through the tight junction barrier.J Cell Biol. 1993 Dec;123(6 Pt 2):1631-3. doi: 10.1083/jcb.123.6.1631. J Cell Biol. 1993. PMID: 8276885 Free PMC article. Review. No abstract available.
Cited by
-
Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni.Infect Immun. 2006 Dec;74(12):6581-9. doi: 10.1128/IAI.00958-06. Epub 2006 Oct 2. Infect Immun. 2006. PMID: 17015453 Free PMC article.
-
Differential effects of testosterone and TGF-β3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier.Exp Cell Res. 2010 Oct 15;316(17):2945-60. doi: 10.1016/j.yexcr.2010.07.018. Epub 2010 Aug 1. Exp Cell Res. 2010. PMID: 20682309 Free PMC article.
-
Polyamines regulate expression of E-cadherin and play an important role in control of intestinal epithelial barrier function.Inflammopharmacology. 2005;13(1-3):91-101. doi: 10.1163/156856005774423890. Inflammopharmacology. 2005. PMID: 16259731 Review.
-
Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics.Proc Natl Acad Sci U S A. 2009 Jun 23;106(25):10213-8. doi: 10.1073/pnas.0901700106. Epub 2009 Jun 9. Proc Natl Acad Sci U S A. 2009. PMID: 19509333 Free PMC article.
-
Bile duct ligation in the rat causes upregulation of ZO-2 and decreased colocalization of claudins with ZO-1 and occludin.Histochem Cell Biol. 2008 Mar;129(3):289-99. doi: 10.1007/s00418-007-0374-7. Epub 2008 Jan 15. Histochem Cell Biol. 2008. PMID: 18197414
References
-
- Balda MS, Gonzalez-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, Garcia-Sainz JA, Cereijido M. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991;122:193–202. - PubMed
-
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical–basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

