In vivo detection of dendritic cell antigen presentation to CD4(+) T cells
- PMID: 9182685
- PMCID: PMC2196354
- DOI: 10.1084/jem.185.12.2133
In vivo detection of dendritic cell antigen presentation to CD4(+) T cells
Abstract
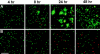
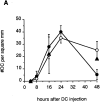
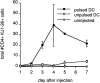
Although lymphoid dendritic cells (DC) are thought to play an essential role in T cell activation, the initial physical interaction between antigen-bearing DC and antigen-specific T cells has never been directly observed in vivo under conditions where the specificity of the responding T cells for the relevant antigen could be unambiguously assessed. We used confocal microscopy to track the in vivo location of fluorescent dye-labeled DC and naive TCR transgenic CD4(+) T cells specific for an OVA peptide-I-Ad complex after adoptive transfer into syngeneic recipients. DC that were not exposed to the OVA peptide, homed to the paracortical regions of the lymph nodes but did not interact with the OVA peptide-specific T cells. In contrast, the OVA peptide-specific T cells formed large clusters around paracortical DC that were pulsed in vitro with the OVA peptide before injection. Interactions were also observed between paracortical DC of the recipient and OVA peptide-specific T cells after administration of intact OVA. Injection of OVA peptide-pulsed DC caused the specific T cells to produce IL-2 in vivo, proliferate, and differentiate into effector cells capable of causing a delayed-type hypersensitivity reaction. Surprisingly, by 48 h after injection, OVA peptide-pulsed, but not unpulsed DC disappeared from the lymph nodes of mice that contained the transferred TCR transgenic population. These results demonstrate that antigen-bearing DC directly interact with naive antigen-specific T cells within the T cell-rich regions of lymph nodes. This interaction results in T cell activation and disappearance of the DC.
Figures







References
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
-
- Tse HY, Schwartz RH, Paul WE. Cell-cell interactions in the T cell proliferative response. J Immunol. 1980;125:401–500. - PubMed
-
- Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

