Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma
- PMID: 9182686
- PMCID: PMC2196351
- DOI: 10.1084/jem.185.12.2143
Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma
Abstract
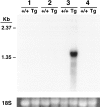
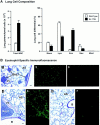
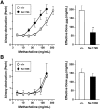
We have generated transgenic mice that constitutively express murine interleukin (IL)-5 in the lung epithelium. Airway expression of this cytokine resulted in a dramatic accumulation of peribronchial eosinophils and striking pathologic changes including the expansion of bronchus-associated lymphoid tissue (BALT), goblet cell hyperplasia, epithelial hypertrophy, and focal collagen deposition. These changes were also accompanied by eosinophil infiltration of the airway lumen. In addition, transgenic animals displayed airway hyperresponsiveness to methacholine in the absence of aerosolized antigen challenge. These findings demonstrate that lung-specific IL-5 expression can induce pathologic changes characteristic of asthma and may provide useful models to evaluate the efficacy of potential respiratory disease therapies or pharmaceuticals.
Figures




References
-
- O'Byrne PM. Allergen-induced airway hyperresponsiveness. J Allergy Clin Immunol. 1988;81:119–127. - PubMed
-
- Empey DW, Laitinen LA, Jacobs L, Gold WM, Nadel JA. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Resp Dis. 1976;113:131–139. - PubMed
-
- McBride DE, Koenig JQ, Luchtel DL, Williams PV, Henderson W., Jr Inflammatory effects of ozone in the upper airways of subjects with asthma. Am J Resp Crit Care Med. 1994;149:1192–1197. - PubMed
-
- Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, Holgate ST, Frew AJ, Howarth PH. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Resp Cell Mol Biol. 1996;14:319–326. - PubMed
-
- Henderson W., Jr Role of leukotrienes in asthma. Ann Allergy. 1994;72:272–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

