On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11(-)/-) mutant mice
- PMID: 9182687
- PMCID: PMC2196355
- DOI: 10.1084/jem.185.12.2157
On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11(-)/-) mutant mice
Abstract
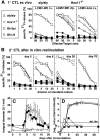
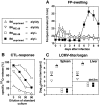
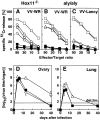
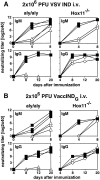
The role of the spleen and of other organized secondary lymphoid organs for the induction of protective antiviral immune responses was evaluated in orphan homeobox gene 11 knockout mice (Hox11(-/-)) lacking the spleen, and in homozygous alymphoplastic mutant mice (aly/aly) possessing a structurally altered spleen but lacking lymph nodes and Peyer's patches. Absence of the spleen had no major effects on the immune response, other than delaying the antibody response by 1-2 d. In aly/aly mice, the thymus-independent IgM response against vesicular stomatitis virus (VSV) was delayed and reduced, whereas the T-dependent switch to the protective IgG was absent. Therefore, aly/aly mice were highly susceptible to VSV infection. Since aly/aly spleen cells yielded neutralizing IgM and IgG after adoptive transfer into recipients with normally structured secondary lymphoid organs, these data suggest that the structural defect was mainly responsible for inefficient T-B cooperation. Although aly/aly mice generated detectable, but reduced, CTL responses after infection with vaccinia virus (VV) and lymphocytic choriomeningitis virus (LCMV), the elimination of these viruses was either delayed (VV) or virtually impossible (LCMV); irrespective of the dose or the route of infection, aly/aly mice developed life-long LCMV persistence. These results document the critical role of organized secondary lymphoid organs in the induction of naive T and B cells. These structures also provide the basis for cooperative interactions between antigen-presenting cells, T cells, and B cells, which are a prerequisite for recovery from primary virus infections via skin or via blood.
Figures






References
-
- Paul, W.E. 1993. Fundamental Immunology. Raven Press, New York. 1490 pp.
-
- Mackay CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423–427. - PubMed
-
- Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. - PubMed
-
- Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. - PubMed
-
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

