Cholecystokinin increases GABA release by inhibiting a resting K+ conductance in hippocampal interneurons
- PMID: 9185537
- PMCID: PMC6573316
- DOI: 10.1523/JNEUROSCI.17-13-04994.1997
Cholecystokinin increases GABA release by inhibiting a resting K+ conductance in hippocampal interneurons
Abstract
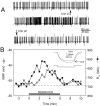
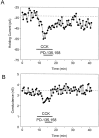
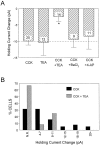
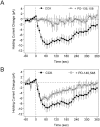
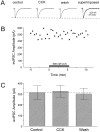
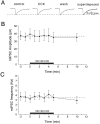
Cholecystokinin (CCK) is found co-localized with the inhibitory neurotransmitter GABA in interneurons of the hippocampus. Also, CCK receptors are found in abundance in this brain region. The possibility that CCK alters interneuron activity was examined using whole-cell current- and voltage-clamp recordings from visualized interneurons in the stratum radiatum of area CA1 in rat hippocampal slices. The effect of CCK on GABA-mediated IPSCs was also determined in pyramidal neurons. The sulfated octapeptide CCK-8S increased action potential frequency or generated inward currents in the majority of interneurons. These effects of CCK persisted in the presence of tetrodotoxin and cadmium, suggesting that they were direct. Current-voltage plots revealed that CCK-8S inhibited a conductance that was linear across command potentials and reversed near the equilibrium potential for K+ ions. The K+ channel blocker tetraethylammonium (10 mM) generated inward currents similar to those initiated by CCK, and it occluded the effect of the peptide. BaCl2 (1 mM) and 4-aminopyridine (2 mM) did not alter the effect of CCK. The CCKB receptor antagonist PD-135,158 completely blocked the inward currents generated by CCK-8S. CCK also resulted in an increase in spontaneous action potential-dependent IPSC frequency, but no changes in action potential-independent miniature IPSCs or evoked IPSCs in pyramidal neurons. These results provide evidence that CCK can depolarize hippocampal interneurons through the inhibition of a resting K+ conductance, leading to increased tonic inhibition of pyramidal neurons. This action of CCK may contribute to its anticonvulsant properties, as observed in limbic seizure models.
Figures


Similar articles
-
Electrophysiological changes in rat hippocampal pyramidal neurons produced by cholecystokinin octapeptide.Neuroscience. 1997 Jun;78(4):1005-16. doi: 10.1016/s0306-4522(96)00653-7. Neuroscience. 1997. PMID: 9174069
-
Membrane properties and synaptic currents evoked in CA1 interneuron subtypes in rat hippocampal slices.J Neurophysiol. 1996 Jul;76(1):1-16. doi: 10.1152/jn.1996.76.1.1. J Neurophysiol. 1996. PMID: 8836204
-
Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurons.J Neurosci. 1996 Jan 15;16(2):572-85. doi: 10.1523/JNEUROSCI.16-02-00572.1996. J Neurosci. 1996. PMID: 8551341 Free PMC article.
-
Bidirectional modulation of GABAergic transmission by cholecystokinin in hippocampal dentate gyrus granule cells of juvenile rats.J Physiol. 2006 Apr 15;572(Pt 2):425-42. doi: 10.1113/jphysiol.2005.104463. Epub 2006 Feb 2. J Physiol. 2006. PMID: 16455686 Free PMC article.
-
Contribution of the hyperpolarization-activated current (I(h)) to membrane potential and GABA release in hippocampal interneurons.J Neurophysiol. 2001 Jul;86(1):261-8. doi: 10.1152/jn.2001.86.1.261. J Neurophysiol. 2001. PMID: 11431507
Cited by
-
Sleep links hippocampal propensity for epileptiform activity to its viscerosensory inputs.Front Neurosci. 2025 Mar 13;19:1559529. doi: 10.3389/fnins.2025.1559529. eCollection 2025. Front Neurosci. 2025. PMID: 40182148 Free PMC article.
-
Brain expression and song regulation of the cholecystokinin gene in the zebra finch (Taeniopygia guttata).J Comp Neurol. 2011 Feb 1;519(2):211-37. doi: 10.1002/cne.22513. J Comp Neurol. 2011. PMID: 21165972 Free PMC article.
-
Cannabinoid disruption of learning mechanisms involved in reward processing.Learn Mem. 2018 Aug 16;25(9):435-445. doi: 10.1101/lm.046748.117. Print 2018 Sep. Learn Mem. 2018. PMID: 30115765 Free PMC article. Review.
-
Inflammatory mediators of opioid tolerance: Implications for dependency and addiction.Peptides. 2019 May;115:51-58. doi: 10.1016/j.peptides.2019.01.003. Epub 2019 Mar 16. Peptides. 2019. PMID: 30890355 Free PMC article. Review.
-
Candidate genes in ocular dominance plasticity.Front Neurosci. 2012 Feb 1;6:11. doi: 10.3389/fnins.2012.00011. eCollection 2012. Front Neurosci. 2012. PMID: 22347157 Free PMC article.
References
-
- Boden PR, Hill RG. Effects of cholecystokinin and pentagastrin on rat hippocampal neurons maintained in vitro. Neuropeptides. 1988;12:95–103. - PubMed
-
- Bohme G, Stutzmann J, Blanchard J. Excitatory effects of cholecystokinin in rat hippocampus: pharmacological response compatible with central- or B-type CCK receptors. Brain Res. 1988;451:309–318. - PubMed
-
- Bohme G, Durieux C, Stutzmann J, Charpentier B, Roques BP, Blanchard J. Electrophysiological studies with new CCK analogs: correlation with binding affinity on B-type receptors. Peptides. 1989;10:407–414. - PubMed
-
- Branchereau P, Champagnat J, Denavit-Saubie M. Cholecystokinin gated currents in neurons of the rat solitary complex in vitro. J Neurophysiol. 1993;70:2584–2595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
