Tyrosine phosphorylation of nicotinic acetylcholine receptor mediates Grb2 binding
- PMID: 9185541
- PMCID: PMC6573310
- DOI: 10.1523/JNEUROSCI.17-13-05038.1997
Tyrosine phosphorylation of nicotinic acetylcholine receptor mediates Grb2 binding
Abstract
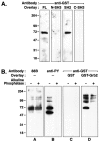
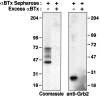
Tyrosine phosphorylation of the nicotinic acetylcholine receptor (AChR) is associated with an altered rate of receptor desensitization and also may play a role in agrin-induced receptor clustering. We have demonstrated a previously unsuspected interaction between Torpedo AChR and the adaptor protein Grb2. The binding is mediated by the Src homology 2 (SH2) domain of Grb2 and the tyrosine-phosphorylated delta subunit of the AChR. Dephosphorylation of the delta subunit abolishes Grb2 binding. A cytoplasmic domain of the delta subunit contains a binding motif (pYXNX) for the SH2 domain of Grb2. Indeed, a phosphopeptide corresponding to this region of the delta subunit binds to Grb2 SH2 fusion proteins with relatively high affinity, whereas a peptide lacking phosphorylation on tyrosine exhibits no binding. Grb2 is colocalized with the AChR on the innervated face of Torpedo electrocytes. Furthermore, Grb2 specifically copurifies with AChR solubilized from postsynaptic membranes. These data suggest a novel role for tyrosine phosphorylation of the AChR in the initiation of a Grb2-mediated signaling cascade at the postsynaptic membrane.
Figures




References
-
- Blaikie P, Immanuel D, Wu J, Li N, Yajnik V, Margolis B. A region in Shc distinct from the SH2 domain can bind tyrosine-phosphorylated growth factor receptors. J Biol Chem. 1994;269:32031–32034. - PubMed
-
- Bowe MA, Deyst KA, Leszyk JD, Fallon JR. Identification and purification of an agrin receptor from Torpedo postsynaptic membranes: a heteromeric complex related to the dystroglycans. Neuron. 1994;12:1173–1180. - PubMed
-
- Campanelli JT, Roberds SL, Campbell KP, Scheller RH. A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell. 1994;77:663–674. - PubMed
-
- Chardin P, Cussac D, Maignan S, Ducruix A. The Grb2 adaptor. FEBS Lett. 1995;369:47–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
